Bicifadine Hydrochloride synthesis
- Product Name:Bicifadine Hydrochloride
- CAS Number:66504-88-9
- Molecular formula:C12H16ClN
- Molecular Weight:209.7151
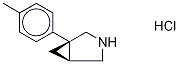
![3-Azabicyclo[3.1.0]hexane, 1-(4-methylphenyl)-, (1S,5R)-](/CAS/20210305/GIF/83213-67-6.gif)
83213-67-6
0 suppliers
inquiry

66504-88-9
11 suppliers
inquiry
Yield:66504-88-9 87%
Reaction Conditions:
with hydrogenchloride in ethyl acetate;isopropyl alcohol at 20;Product distribution / selectivity;
Steps:
IV.C.2
Method 2 EPO
References:
WO2006/96810,2006,A2 Location in patent:Page/Page column 66-67