Pseudouridine synthesis
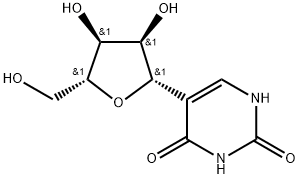
- Product Name:Pseudouridine
- CAS Number:1445-07-4
- Molecular formula:C9H12N2O6
- Molecular Weight:244.2
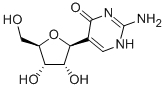
57100-18-2
34 suppliers
$75.00/5 mg

1445-07-4
254 suppliers
$55.00/2.5mg
Yield:1445-07-4 3.2 g (85%)
Reaction Conditions:
with acetic anhydride in pyridine;ethyl acetate
Steps:
30 Peracetylated Pseudoisocytidine 2
Pseudoisocytidine (1) (2.5 g, 9 mmoles) was dissolved in 40 ml dry pyridine. Acetic anhydride (8.5 ml, 90 mmoles) was added and the mixture was stirred under argon for at least 4 hours at room temperature. The reaction can be monitored by HPLC (C18 column, buffer A: 0.1M TEAA, pH 7.5; buffer B: acetonitrile; gradient: 5-95%B over 20 minutes). The pyridine was removed under vacuum and the residual oil was dissolved in 500 ml of ethyl acetate. More ethyl acetate may be added to get a clear solution since the product has limited solubility in ethyl acetate. The organic phase was washed three times with brine and dried over anhydrous Na2SO4, filtered and the solvent removed. The white solid was recrystallized from ethyl acetate/hexane yielding 3.2 g (85%) of 2.
References:
Affymetrix, INC. US2005/3496, 2005, A1
![2,4(1H,3H)-Pyrimidinedione, 5-[2,3-O-(1-methylethylidene)-β-D-ribofuranosyl]-](/CAS/20210305/GIF/28113-58-8.gif)
28113-58-8
1 suppliers
inquiry

1445-07-4
254 suppliers
$55.00/2.5mg
![D-Ribitol, 1,4-anhydro-1-C-[2,4-bis(1,1-dimethylethoxy)-5-pyrimidinyl]-5-O-(1-methoxy-1-methylethyl)-2,3-O-(1-methylethylidene)-, (1S)-](/CAS/20240320/GIF/631920-69-9.gif)
631920-69-9
0 suppliers
inquiry

1445-07-4
254 suppliers
$55.00/2.5mg
![2,4(1H,3H)-Pyrimidinedione, 5-[5-O-[(1,1-dimethylethyl)diphenylsilyl]-2,3-O-(1-methylethylidene)-β-D-ribofuranosyl]-](/CAS/20210305/GIF/1024616-70-3.gif)
1024616-70-3
1 suppliers
inquiry

1445-07-4
254 suppliers
$55.00/2.5mg
68168-19-4
0 suppliers
inquiry

1445-07-4
254 suppliers
$55.00/2.5mg