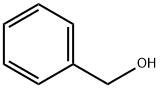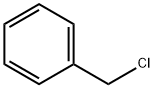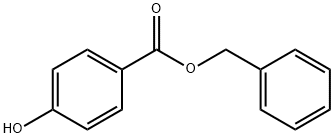
Benzylparaben synthesis
- Product Name:Benzylparaben
- CAS Number:94-18-8
- Molecular formula:C14H12O3
- Molecular Weight:228.24
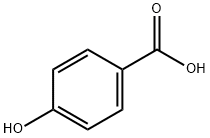
99-96-7
814 suppliers
$5.00/10g

94-18-8
278 suppliers
$12.00/25g
Yield:94-18-8 111.0 g (yield 94.%)
Reaction Conditions:
with di(n-butyl)tin oxide;benzyl alcohol
Steps:
1 Example 1
Example 1 69.1 g (0.5 mole) of p-hydroxybenzoic acid, 216.2 g (2 moles) of benzyl alcohol and 1.4 g of dibutyltin oxide were fed into a four-necked glass flask, and reacted at 200° C. for 6 hours with stirring in an atmosphere of nitrogen. Water formed by the reaction and a small amount of phenol resulting from the decomposition of the p-hydroxybenzoic acid were distilled off from the reaction system. After the reaction, the excess of benzyl alcohol was evaporated under reduced pressure to give 111.0 g (yield 94.%) of crude benzyl p-hydroxybenzoate having a purity of 97.4%.
References:
Kabushiki Kaisha Veno Seiyaku Oyo Kenkyuo US4973737, 1990, A
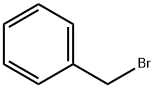
100-39-0
439 suppliers
$10.00/10 g
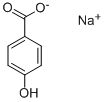
114-63-6
215 suppliers
$7.00/10g

94-18-8
278 suppliers
$12.00/25g