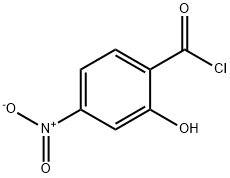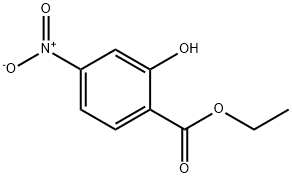
Benzoic acid, 2-hydroxy-4-nitro-, ethyl ester synthesis
- Product Name:Benzoic acid, 2-hydroxy-4-nitro-, ethyl ester
- CAS Number:78987-51-6
- Molecular formula:C9H9NO5
- Molecular Weight:211.17
Yield:78987-51-6 89%
Reaction Conditions:
with thionyl chloride at 0 - 20;Product distribution / selectivity;Heating / reflux;
Steps:
1.c; 2.c; 3.c; 4.d
c. Synthesis of intermediate E; To a solution of anhydrous ethanol (500 ml) was charged D (50 g, 0.27 mol) and the reaction mixture was cooled to 0-5 0C at which time thionyl chloride (59.6 ml, 0.81 mol) was slowly added to keep the reaction mixture temperature < 20 0C. After complete thionyl chloride addition the reaction mixture was heated to reflux and maintained at reflux overnight. The reaction mixture was cooled to 20 0C and then concentrated to an oil by evaporation under reduced pressure. To the residue was charged a mixture of water (200 ml) and ethyl acetate (200 ml). The layers were separated and the aqueous was extracted with additional ethyl acetate (2 x 200 ml). The combined organic layers were washed with 10% sodium bicarbonate solution (200 ml), water (200 ml) and brine (200 ml) and then dried over sodium sulfate, filtered and concentrated under reduced pressure to give E as a yellow solid (51 g, 89%).; c. Synthesis of Intermediate E; To a solution of anhydrous ethanol (250 ml) was charged intermediate D (25 g, 0.137 mol) and the reaction mixture was cooled to 0-5 C at which time thionyl chloride (29.8 ml, 0.41 mol) was slowly added to keep the reaction mixture temperature < 20 C. After complete thionyl chloride addition the reaction mixture was heated to reflux and maintained at reflux overnight. The reaction mixture was cooled to 20 C and then concentrated to an oil by evaporation under reduced pressure. To the residue was charged a mixture of water (100 ml) and ethyl acetate (100 ml). The layers were separated and the aqueous was extracted with additional ethyl acetate (2 x 100 ml). The combined organic layers were washed with 10% sodium bicarbonate solution (100 ml), water (100 ml) and brine (100 ml) and then dried over sodium sulfate, filtered and concentrated under reduced pressure to give intermediate E as a yellow solid (27 g, 93%).; c. Synthesis of intermediate E; To a solution of anhydrous ethanol (500 ml) was charged intermediate D (50 g, 0.27 mol) and the reaction mixture was cooled to 0-5 C at which time thionyl chloride (59.6 ml, 0.81 mol) was slowly added to keep the reaction mixture temperature < 20 C. After complete thionyl chloride addition the reaction mixture was heated to reflux and maintained at reflux overnight. The reaction mixture was cooled to 20 C and then concentrated to an oil by evaporation under reduced pressure. To the residue was charged a mixture of water (200 ml) and ethyl acetate (200 ml). The layers were separated and the aqueous was extracted with additional ethyl acetate (2 x 200 ml). The combined organic layers were washed with 10% sodium bicarbonate solution (200 ml), water (200 ml) and brine (200 ml) and then dried over sodium sulfate, filtered and concentrated under reduced pressure to give intermediate E as a yellow solid (51 g, 89%).; d. Synthesis of common intermediate G; To a solution of a protic polar solvent, such as ethanol, is charged 2-hydroxy- 4-nitrobenzoic acid followed by a chlorinating agent such as thionyl chloride. After reflux for 8-16 hours the reaction mixture is concentrated and extracted with an organic solvent such as ethyl acetate, washed with sodium carbonate solution, water and brine and the organic evaporated in vacuo to afford the product as a yellow solid.
References:
WO2008/11539,2008,A2 Location in patent:Page/Page column 36; 40; 44; 48
99-77-4
219 suppliers
$12.00/1mg

78987-51-6
5 suppliers
inquiry
619-19-2
170 suppliers
$9.00/1g

78987-51-6
5 suppliers
inquiry