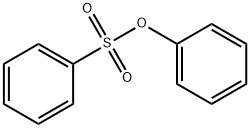
BENZENESULFONIC ACID PHENYL ESTER synthesis
- Product Name:BENZENESULFONIC ACID PHENYL ESTER
- CAS Number:4358-63-8
- Molecular formula:C12H10O3S
- Molecular Weight:234.27
Yield:4358-63-8 97%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 6 h;Inert atmosphere;
Steps:
General procedure for the preparation of aryl benzenesulfonates
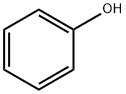
To a stirred solution of phenol (1a) (5.00 g, 53.1 mmol) in CH2Cl2 (100 mL) at 0 °C, benzenesulfonyl chloride (8.21 mL, 63.8 mmol) and Et3N (8.83 mL, 63.8 mmol) were added. The reaction mixture was warmed to room temperature and stirred for 6 h. The mixture was quenched with 1 M HCl solution, extracted with CH2Cl2, dried over Na2SO4, and concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography (hexane:EtOAc 9:1 3:1) to give phenyl benzenesulfonate 2a (12.1 g, 51.7 mmol) in 97% yield as colorless viscous liquid. Data[20]: 1H NMR (CDCl3, 400 MHz) δ 7.82 (d, J 7.2 Hz, 2H), 7.66 (t, J 6.4 Hz, 1H), 7.51 (t, J 7.6 Hz, 2H), 7.30-7.22 (m, 3H), 6.96 (d, J 7.6 Hz, 2H) ppm.
References:
Alam, Mohammad Shariful;Koo, Sangho [Synthetic Communications,2018,vol. 48,# 3,p. 247 - 254] Location in patent:supporting information
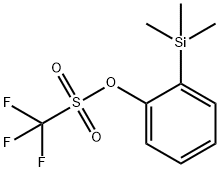
88284-48-4
172 suppliers
$9.00/250mg
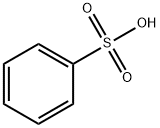
98-11-3
394 suppliers
$19.89/100g

4358-63-8
20 suppliers
inquiry
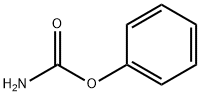
622-46-8
98 suppliers
$33.20/5g
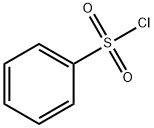
98-09-9
359 suppliers
$12.00/25g
98-10-2
347 suppliers
$18.00/25g

4358-63-8
20 suppliers
inquiry