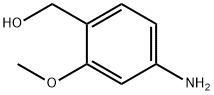
Benzenemethanol, 4-amino-2-methoxy- synthesis
- Product Name:Benzenemethanol, 4-amino-2-methoxy-
- CAS Number:1261873-18-0
- Molecular formula:C8H11NO2
- Molecular Weight:153.18
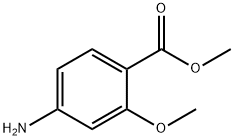
27492-84-8
318 suppliers
$6.00/5g

1261873-18-0
19 suppliers
inquiry
Yield:-
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 65;
Steps:
19 Synthesis of compound 2-A
Lithium aluminum hydride (228mg) was added to 15mL tetrahydrofuran,Dissolve methyl 2-methoxy-4-aminobenzoate (725 mg) in 5 mL of tetrahydrofuran at 0°C and slowly drop it into the reaction solution.After the dropwise addition is complete, move to 65°C and reflux and stir to react. Overnight TLC monitors the completion of the reaction,Add 1mL water dropwise at 0, quench with 1mL 0.5N sodium hydroxide solution, filter out the insoluble matter with Celite, The filtrate was purified by concentrated column chromatography (petroleum ether: ethyl acetate=1:1) to obtain compound 2-A (420 mg).
References:
China Pharmaceutical University;Jiang Sheng;Hao Haiping;Wang Tianyu;Wang Minmin;Zhang Wanheng;Ni Yong;Qiu Yatao;Wu Xiaoxing CN111718310, 2020, A Location in patent:Paragraph 0118-