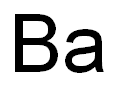
Barium synthesis
- Product Name:Barium
- CAS Number:7440-39-3
- Molecular formula:Ba
- Molecular Weight:137.33
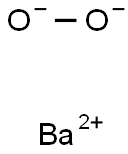
1304-29-6
0 suppliers
$13.44/50g
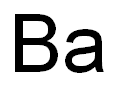
7440-39-3
149 suppliers
$42.63/50ml
Yield:-
Reaction Conditions:
with Ti or Zr or Hf or Th;oxides of La or of Ce or of Th in neat (no solvent);reduction of BaO2 with Ti or Zr or Hf or Th dild. with oxides of La or of Ce or of Th;;
References:
[Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Ba: SVol., 10, page 169 - 172] FR868299,1941,A [C. I,1942,p. 2049]