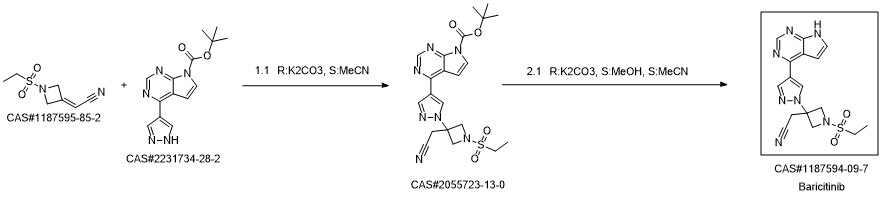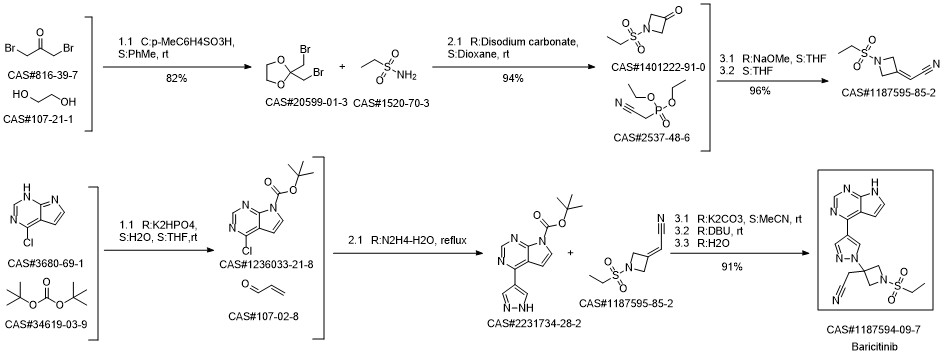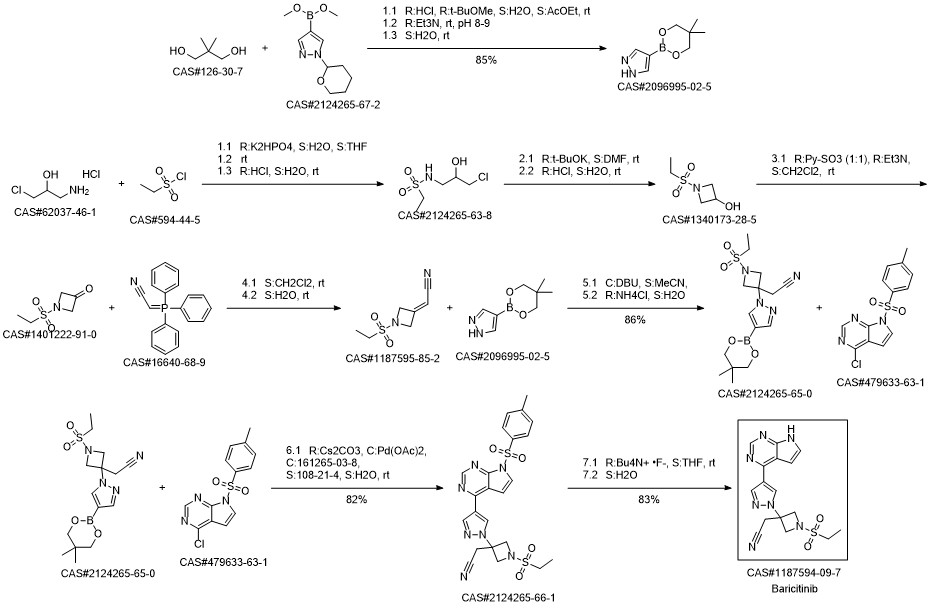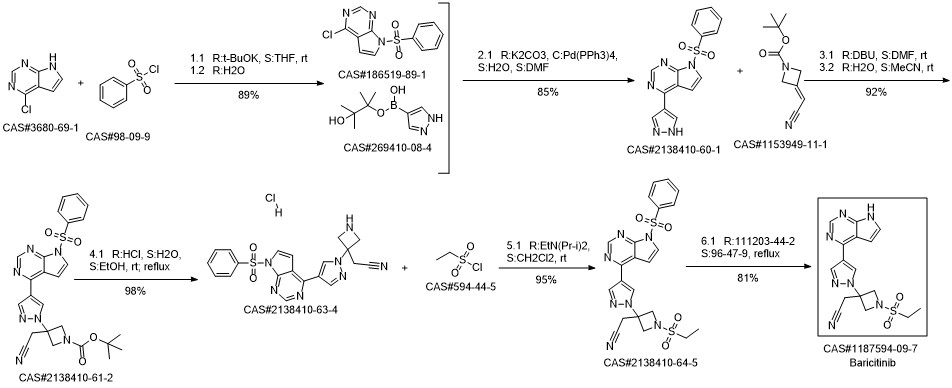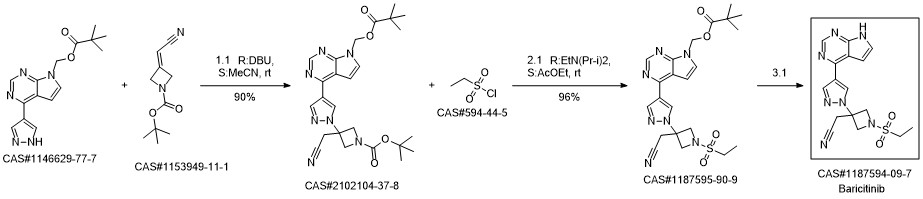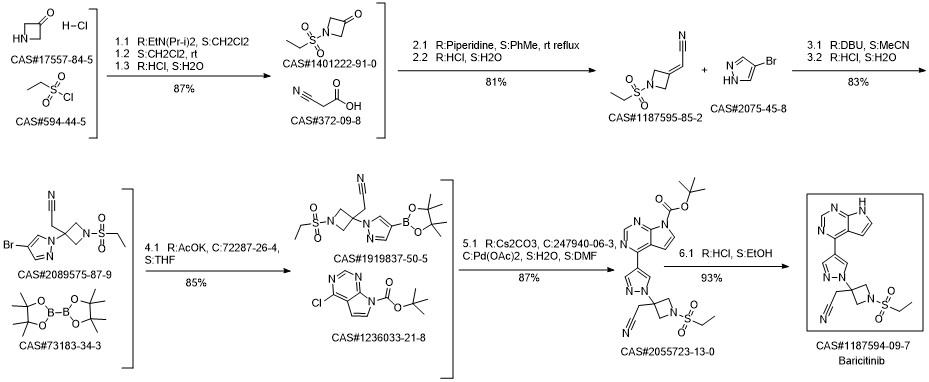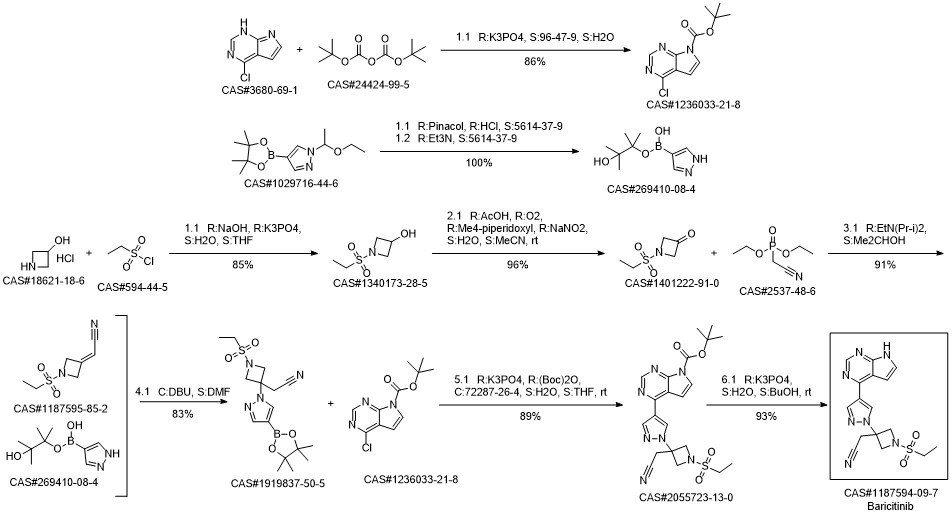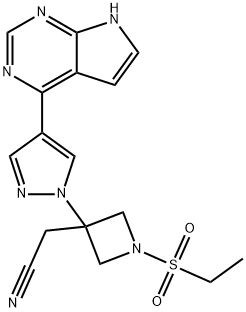
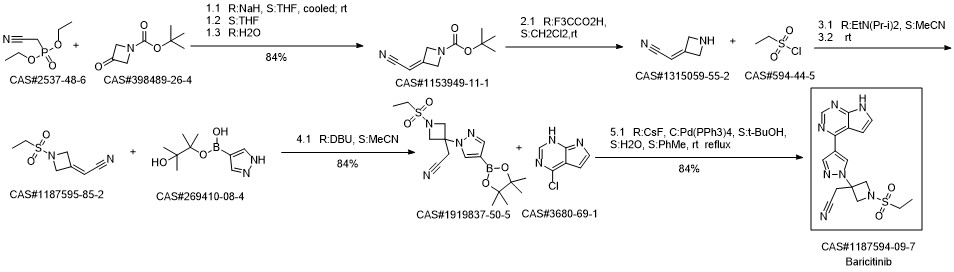
Baricitinib synthesis
- Product Name:Baricitinib
- CAS Number:1187594-09-7
- Molecular formula:C16H17N7O2S
- Molecular Weight:371.42
Reference: Xu, Jiaojiao; Cai, Jin; Chen, Junding; Zong, Xi; Wu, Xuan; Ji, Min; Wang, Peng. An efficient synthesis of baricitinib. Journal of Chemical Research. Volume 40. Issue 4. Pages 205-208. Journal. (2016).
![2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile](/CAS/20180531/GIF/1187594-13-3.gif)
1187594-13-3
60 suppliers
inquiry

1187594-09-7
486 suppliers
$38.00/25mg
Yield:1187594-09-7 83.8%
Reaction Conditions:
Stage #1:2-[1-ethanesulfonyl-3-[4-(7-[(2-(trimethylsilyl)ethoxy)methyl]-7H-pyrrolo[2,3-d]pyrimidine-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl]acetonitrile with lithium tetrafluoroborate;water in acetonitrile at 75;
Stage #2: with ammonium hydroxide;water in acetonitrile; pH=9 - 10 at 0 - 10;Product distribution / selectivity;
Steps:
61.5
To a solution of 2-(1-(ethylsulfonyl)-3-(4-(7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile (6, 327 g, 655 mmol) in acetonitrile (3 L) and water (300 mL) was added LiBF4 (614 g, 6.55 mol, 10.0 equiv). The resulting reaction mixture was stirred at 75° C. for overnight. The reaction mixture was cooled to 0° C. before a solution of ammonium hydroxide (NH4OH, 570 mL) in water (2.2 L) was added slowly to keep the temperature below 10° C. (pH 9-10). The mixture was stirred at room temperature for overnight. When the reaction was deemed complete, water (10 L) was added and the resulting mixture was vigorously stirred for 3 h at room temperature. The solids were collected by filtration, washed with water (6.7 L) and heptane (6.7 L), and dried in vacuum oven at 45° C. over the weekend. The dried solid was then dissolved in 20% MeOH in dichloromethane (12 L), and was purified by column chromatography on 1.3 Kg of silica gel eluting with a 20% MeOH in dichloromethane solution (18L) to afford 2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(ethylsulfonyl)azetidin-3-yl)acetonitrile (7, 204 g, 243.3 g theoretical, 83.8% yield) as an off-white solid. For 7: 1H NMR (300 MHz, d6-DMSO) δ 1.25 (t, 3H), 3.25 (q, 2H), 3.75 (s, 2H), 4.25 (d, 2H), 4.65 (d, 2H), 7.10 (d, 1H), 7.65 (dd, 1H), 8.50 (s, 1H), 8.70 (s, 1H), 8.95 (s, 1H), 12.2 (bs, 1H); MS: m/z calcd. 372.12; found: 372.0.
References:
INCYTE CORPORATION US2009/233903, 2009, A1 Location in patent:Page/Page column 58; 60
![(4-(1-(3-(cyanomethyl)-1-(ethylsulfonyl)azetidin-3-yl)-1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl pivalate](/CAS/20200331/GIF/1187595-90-9.gif)
1187595-90-9
60 suppliers
inquiry

1187594-09-7
486 suppliers
$38.00/25mg
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
845 suppliers
$6.00/1g

1187594-09-7
486 suppliers
$38.00/25mg
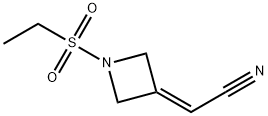
1187595-85-2
277 suppliers
inquiry

1187594-09-7
486 suppliers
$38.00/25mg
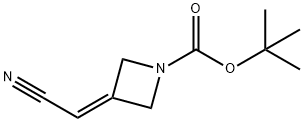
1153949-11-1
195 suppliers
$14.00/1g

1187594-09-7
486 suppliers
$38.00/25mg