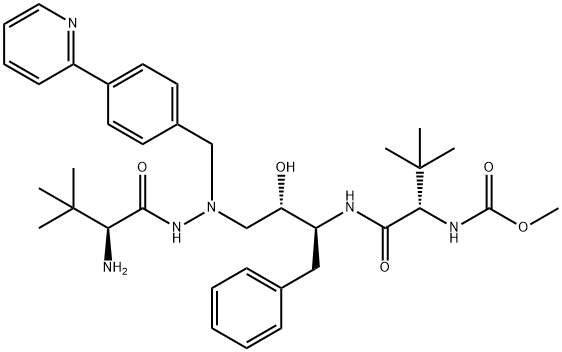
Atazanavir Impurity 8 synthesis
- Product Name:Atazanavir Impurity 8
- CAS Number:1028634-76-5
- Molecular formula:C36H50N6O5
- Molecular Weight:646.82
![methyl N-(1-{[4-(2-{[(benzyloxy)carbonyl]amino}-3,3-dimethyl-N'-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido)-3-hydroxy-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl)carbamate](/CAS/20200515/GIF/1028634-75-4.gif)
1028634-75-4
2 suppliers
inquiry

1028634-76-5
20 suppliers
inquiry
Yield:-
Reaction Conditions:
with ammonium formate;5%-palladium/activated carbon in methanol at 40; for 2 h;
Steps:
5
Example 5 - Preparation of compound (8); Compound 7 (12.2 g), obtained as described in example 4, was suspended in methanol (90 mL), then added with 1.33 g of Pd-C 5% and the suspension was heated to reflux. The mixture was then cooled to 40°C, and a solution of 4.93 g of ammonium formate in methanol/water was added over about one hour. The reaction was heated at 40°C for about one hour, then cooled to about 25-30°C. The suspension was filtered and the filtrate was evaporated in vacuo to a solid residue, which was added with 100 mL of EtOAc and washed with a 5% aqueous solution of NaHCO3. The organic phase was dried over anhydrous sodium sulphate, filtered, and evaporated in vacuo to yield 9.47 of compound 8 as a white solid, which was used for the next step without further purification.
References:
EP1930324,2008,A1 Location in patent:Page/Page column 7