
ANANDAMIDE synthesis
- Product Name:ANANDAMIDE
- CAS Number:94421-68-8
- Molecular formula:C22H37NO2
- Molecular Weight:347.53
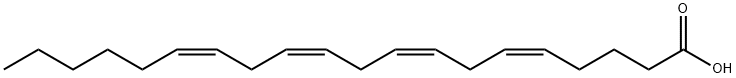
506-32-1
446 suppliers
$15.00/5g

141-43-5
903 suppliers
$9.00/10g

94421-68-8
100 suppliers
$32.00/5mg
Yield:94421-68-8 96%
Reaction Conditions:
Stage #1:all cis 5,8,11,14-eicosatetraenoic acid with 1,1'-carbonyldiimidazole in dichloromethane at 20; for 0.5 h;
Stage #2:ethanolamine in dichloromethane for 12 h;
Steps:
(21) General Procedure for Amide Coupling
General procedure: To a stirred solution of the fatty acid (1.0 mmol, 1.0 equiv.) inCH2Cl2 (5 mL) was added CDI (0.178 g, 1.1 mmol, 1.1 equiv.).After 30 min at room temperature, the amine (1.1 mmol, 1.1equiv.) was added. After 12 h, CH2Cl2 (25 mL) was added, followedby saturated aqueous NH4Cl. The mixture was acidified topH 2 by addition of HCl, the organic phase was separated, andthe aqueous layer was further extracted with CH2Cl2 (3 × 10mL). The organic phases were combined, dried over Na2SO4, filtered,and concentrated in vacuo, to give the amide.
References:
Johansson, Silje J. R.;Johannessen, Tonje;Ellefsen, Christiane F.;Ristun, Mali S.;Antonsen, Simen;Hansen, Trond V.;Stenstrom, Yngve;Nolsoe, Jens M. J. [Synlett,2019,vol. 30,# 2,art. no. ST-2018-D0672-L,p. 213 - 217] Location in patent:supporting information

141-43-5
903 suppliers
$9.00/10g
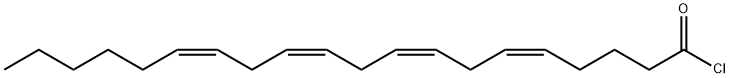
57303-04-5
23 suppliers
$25.00/1mg

94421-68-8
100 suppliers
$32.00/5mg
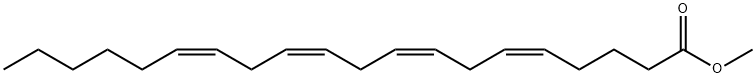
2566-89-4
106 suppliers
$29.00/25mg

141-43-5
903 suppliers
$9.00/10g

94421-68-8
100 suppliers
$32.00/5mg

506-32-1
446 suppliers
$15.00/5g

141-43-5
903 suppliers
$9.00/10g
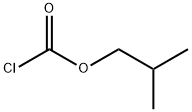
543-27-1
268 suppliers
$10.00/5g

94421-68-8
100 suppliers
$32.00/5mg

506-32-1
446 suppliers
$15.00/5g

94421-68-8
100 suppliers
$32.00/5mg