
Aminoguanidine bicarbonate synthesis
- Product Name:Aminoguanidine bicarbonate
- CAS Number:2582-30-1
- Molecular formula:C2H8N4O3
- Molecular Weight:136.11
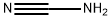
420-04-2
379 suppliers
$15.00/25g

124-38-9
129 suppliers
$214.00/14L

2582-30-1
383 suppliers
$18.00/25g
Yield:2582-30-1 96%
Reaction Conditions:
Stage #1: carbon dioxidewith water;hydrazine at 20 - 55; pH=7 - 11; for 1 h;
Stage #2: CYANAMID;carbon dioxide in water at 65; pH=7; for 4 h;Product distribution / selectivity;
Steps:
2, 4, 5
The process is performed as described in Example 1, except that the aqueous hydrazine hydrate solution is maintained at 55° C. instead of 40° C. and that, during the addition of the cyanamide, the reaction medium is brought to 65° C. and is maintained at this temperature for 4 hours.After drying, 261.1 g of crystals in the form of platelets (photograph No. 2) are obtained with a purity of 99.6%. The crude yield relative to the cyanamide is 96%.; Example 4 Example 2 is repeated on the industrial scale, using a 15 m3 reactor.After spin-filtering for 3 hours, the platelets contain a moisture content of 20% and, at the end of the spin-filtering, the platelets have a mean diameter of 70 μm with a very broad particle size distribution with 20% of the population of particles having a diameter of less than 20 μm.; Example 5 The procedure described in Example 1 is repeated, except that the duration of addition of the cyanamide is 5 hours instead of 2 hours and the duration of the reaction after the addition is reduced from 8 to 5 hours.The yield and also the purity of the AGB crystals obtained are similar to those of Example 1. However, the crystals are rather in the form of platelets (photograph No. 3) and the spin-filtering time is longer.
References:
US6884912,2005,B1 Location in patent:Page/Page column 3
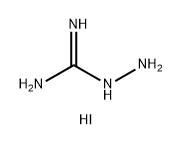
133082-93-6
0 suppliers
inquiry

298-14-6
481 suppliers
$6.00/100g

2582-30-1
383 suppliers
$18.00/25g
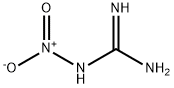
556-88-7
133 suppliers
$38.39/100g

2582-30-1
383 suppliers
$18.00/25g