Altrenogest synthesis
- Product Name:Altrenogest
- CAS Number:850-52-2
- Molecular formula:C21H26O2
- Molecular Weight:310.44
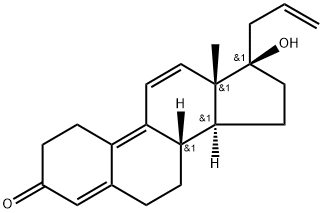
5571-36-8
321 suppliers
$5.00/250mg

1730-25-2
202 suppliers
$66.20/100ml

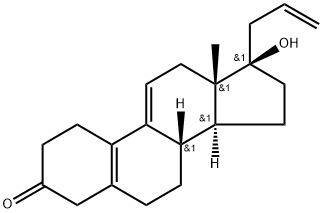
850-52-2
419 suppliers
$28.00/1unit
Yield:850-52-2 78.7 %
Reaction Conditions:
Stage #1: ethylene deltenone;allylmagnesium bromide in 2-methyltetrahydrofuran at 20;
Stage #2: with trifluoroacetic acid in 2-methyltetrahydrofuran at 40 - 50;
Stage #3: with 2,3-dicyano-5,6-dichloro-p-benzoquinone in 2-methyltetrahydrofuran at 20;
Steps:
1-3
Slowly add 16g of DDQ to the filtrate, after the addition is complete, stir for 1 hour, and TLC detects that the reaction of the raw materials is complete.Add 100ml of 10% sodium carbonate solution, stir for 10 minutes, let stand for stratification, add 100ml of water to the organic layer, wash twice, stir for 10 minutes, let stand for liquid separation, add 20g of anhydrous sodium sulfate to the organic layer, dry for 15 minutes, filter, and the filtrate Concentrate to dryness to obtain the crude product; add 100ml acetone to the crude product, stir and dissolve, slowly pour into 200ml water, separate out a large amount of solid, filter to obtain the crude product of 16.5g light yellow allyl progesterone, molar yield (based on 3-ketal ): 83.6%.The crude product was dissolved in 40ml of acetone, and then 20ml of water was added to precipitate a solid, light yellow solid, which was filtered and dried to obtain 13g of the finished product. The molar yield (based on 3-ketal): 78.7%.
References:
CN115109111,2022,A Location in patent:Paragraph 0045-0051; 0052-0055
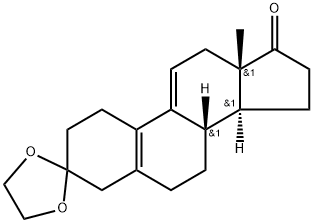
23760-34-1
4 suppliers
inquiry

850-52-2
419 suppliers
$28.00/1unit
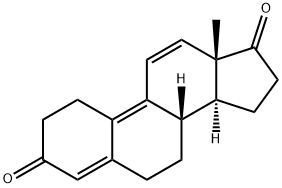
4642-95-9
57 suppliers
$28.00/1mg

1730-25-2
202 suppliers
$66.20/100ml

850-52-2
419 suppliers
$28.00/1unit
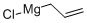
2622-05-1
156 suppliers
$40.00/500mg

4642-95-9
57 suppliers
$28.00/1mg

850-52-2
419 suppliers
$28.00/1unit
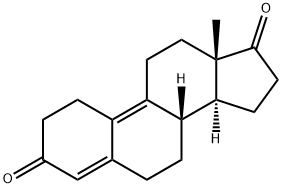
5173-46-6
251 suppliers
$69.00/5g

850-52-2
419 suppliers
$28.00/1unit