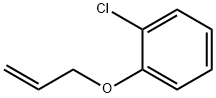
ALLYL 2-CHLOROPHENYL ETHER synthesis
- Product Name:ALLYL 2-CHLOROPHENYL ETHER
- CAS Number:20788-42-5
- Molecular formula:C9H9ClO
- Molecular Weight:168.62
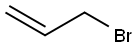
106-95-6
434 suppliers
$10.00/5g
35535-81-0
3 suppliers
inquiry

20788-42-5
13 suppliers
$28.60/250mg
Yield:20788-42-5 99%
Reaction Conditions:
with potassium carbonate in water;acetonitrile at 50; for 48 h;Reagent/catalyst;
Steps:
Synthesis under batch conditions
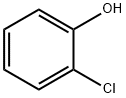
2-Chlorophenol (2) (1.61 mL, 15.6 mmol, 1.0 eq.) and potassiumcarbonate (4.30 g, 31.1 mmol, 2.0 eq.) were dissolved in acetonitrile (60 mL). Allyl bromide (2.70 mL, 31.1 mmol, 2.0 eq.) was then slowly added. The reaction mixture was stirred for two days at 50°C. The excess salt was filtered off and the solvent was removed under reduced pressure. The residuewas taken up into diethyl ether and a saturated NaHCO3 solution. The aqueous phase was separatedfrom the organic phase and extracted twice with approx. 20 mL diethylether. The combined organicphases were dried over magnesium sulfate, filtered and the solvent was removed under reducedpressure. Allylphenyl ether 10 (2.62 g, 15.5 mmol; 99 %) was obtained as a colourless oil.1H-NMR (400 MHz, CDCl3, CHCl3 = 7.26 ppm): [ppm] = 7.37 (1H, dd, J = 7.8 Hz, J = 1.7 Hz,arom.), 7.22-7.17 (1H, m, arom.), 6.94-6.88 (2H, m, arom.), 6.12-6.03 (1H, m, H-2), 5.47 (1H, dq, J =17.3 Hz, J = 1.7 Hz, H-1), 5.31 (1H, dq, J = 10.4 Hz, J = 1.4 Hz, H-1), 4.62 (2H, dt, J = 5.3 Hz, J =1.6 Hz, H-3); 13C-NMR (100 MHz, CDCl3, CDCl3 = 77.16 ppm): [ppm] = 154.1 (q, C-5), 132.7 (t,C-2), 130.4 (t, arom.), 127.6 (t, arom.), 123.1 (q, C-10), 121.5 (t, arom.), 117.9 (s, C-1), 113.8 (t,arom.), 69.7 (s, C-3);Error Bookmark not defined. HRMS (EI): m/z calculated for C9H9OCl:168.0342; found 168.0340; Rf (9:1 pentane/Et2O): 0.62.
References:
Oltmanns, Mona;Kirschning, Andreas [Synlett,2020,vol. 31,# 19,p. 1942 - 1946] Location in patent:supporting information
95-57-8
358 suppliers
$10.00/5g

106-95-6
434 suppliers
$10.00/5g

20788-42-5
13 suppliers
$28.60/250mg

35535-81-0
3 suppliers
inquiry

20788-42-5
13 suppliers
$28.60/250mg
![Benzene, 1-chloro-2-[(1Z)-1-propen-1-yloxy]-](/CAS/20210305/GIF/51896-46-9.gif)
51896-46-9
0 suppliers
inquiry

20788-42-5
13 suppliers
$28.60/250mg
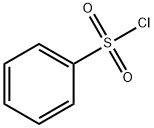
98-09-9
359 suppliers
$12.00/25g

20788-42-5
13 suppliers
$28.60/250mg