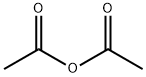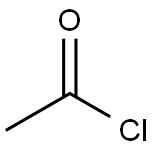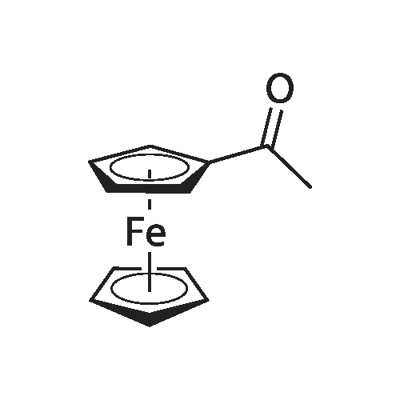
Acetylferrocene synthesis
- Product Name:Acetylferrocene
- CAS Number:1271-55-2
- Molecular formula:C12H12FeO10*
- Molecular Weight:228.07
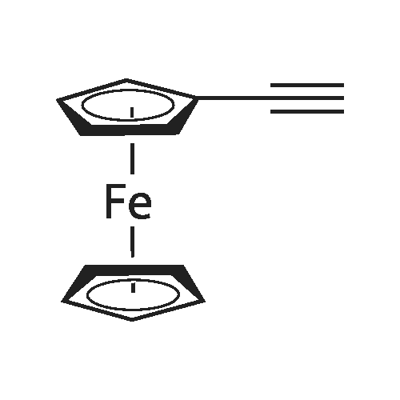
1271-47-2
129 suppliers
$12.00/250mg
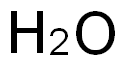
7732-18-5
488 suppliers
$12.69/100ml

1271-55-2
221 suppliers
$8.00/1g
Yield:1271-55-2 83%
Reaction Conditions:
in methanol at 150; under 8250.83 Torr; for 14 h;Autoclave;Inert atmosphere;Green chemistry;
Steps:
The general procedure of the reaction
General procedure: In a 100 mL capacity of autoclave vessel a 60 mL solution of methanol and water (1:2) was added, further 1 mmol alkynes were added to this solution. The autoclave was three times purged withthe gas and then nally pressurized up to the 11 bar pressure. The reaction mixture was vigorously stirred at 150 °C for continuous 14 h. After the completion of the reaction, the reactor was cooled to room temperature, and then the argon pressure was carefully released to the atmospheric pressure. Methanol from the reaction mixture is removed using rotatory evaporator. After that, the reaction mixture was transferred in a separating funnel, and it wasworked up with ethyl acetate. The organic layer was separatedand dried over Na2SO4. Afterwards, it was filtered and concentrated under reduced pressure. The resulted crude mixture waspuried by silica gel column chromatography using ethyl acetate/n-hexane as eluent, and pure keto product was isolated.
References:
Ali, Munsaf;Srivastava, Avinash K.;Joshi, Raj K. [Tetrahedron Letters,2018,vol. 59,# 21,p. 2075 - 2078]

1271-47-2
129 suppliers
$12.00/250mg

1271-55-2
221 suppliers
$8.00/1g
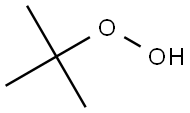
75-91-2
204 suppliers
$26.00/100g
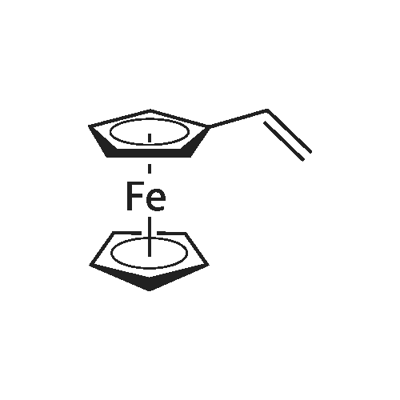
1271-51-8
165 suppliers
$11.00/250mg

1271-55-2
221 suppliers
$8.00/1g