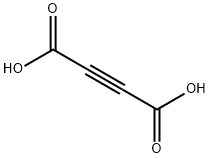
Acetylenedicarboxylic acid synthesis
- Product Name:Acetylenedicarboxylic acid
- CAS Number:142-45-0
- Molecular formula:C4H2O4
- Molecular Weight:114.06

110-65-6
325 suppliers
$11.00/25g

142-45-0
243 suppliers
$10.00/5g
Yield:142-45-0 71%
Reaction Conditions:
with sodium hydroxide;sodium hypochlorite;4-acetylamino-2,2,6,6-tetramethyl-1-piperidinoxy in water; pH=8.5 - 10 at 5 - 10;
Steps:
8 Example 8; Oxydation of 2-butine-1,4-diol to acetylene-dicarboxylic acid in an aqueous monophasic system using acetamido-TEMPO
[0186] 14.4 g (167 mmol) of 2-butyn-1,4-diol are dissolved together with 2.14 g (10.0 mmol) of 4-acetamido-TEMPO in 94 ml of water (reaction component 1). [0187] 6.68 g (167 mmol) of NaOH are dissolved in 337 ml (0.741 mol) of sodium hypochlorite solution (approx. 2.2 M technical bleaching liquor; pH 14) and cooled to 5° C. (reaction component 2). [0188] A flask equipped with mechanical stirrer is initially charged with 50 ml of water and cooled to 3° C. Reaction components 1 and 2 are added in parallel with good stirring and cooling in such a way that the internal temperature does not rise above 10° C. During this time, the pH of the reaction mixture is kept in the range from 8.5 to 10 by adding 20% sodium hydroxide solution. A total of approx. 15 ml of sodium hydroxide solution are consumed. [0189] On completion of addition, stirring is continued for another 20 min. [0190] The reaction mixture contains 11.4 g of acetylene-dicarboxylic acid in solution and 2.1 g of acetylene-dicarboxylic acid in the precipitate formed (overall yield 13.5 g, 71%). Extraction is effected using 300 ml of MTBE, then the pH of the aqueous reaction mixture is adjusted to pH 0 with stirring in an ice bath using conc. sulfuric acid, and extraction is effected by shaking 3 times using 100 ml of MTBE each time. The MTBE extracts of the acidic reaction mixture are concentrated by evaporation. 11.1 g of acetylenedicarboxylic acid are obtained in the form of a colorless solid.
References:
Consortium Fur Elektrochemische Industrie GmbH US2004/59154, 2004, A1 Location in patent:Page 8-9
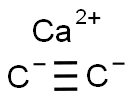
75-20-7
160 suppliers
$47.79/500g

142-45-0
243 suppliers
$10.00/5g
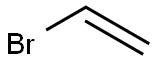
593-60-2
144 suppliers
$25.00/25g

124-38-9
129 suppliers
$175.00/23402

142-45-0
243 suppliers
$10.00/5g
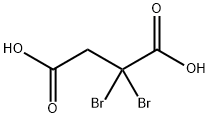
101349-74-0
0 suppliers
inquiry

142-45-0
243 suppliers
$10.00/5g
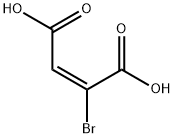
584-99-6
3 suppliers
inquiry

142-45-0
243 suppliers
$10.00/5g