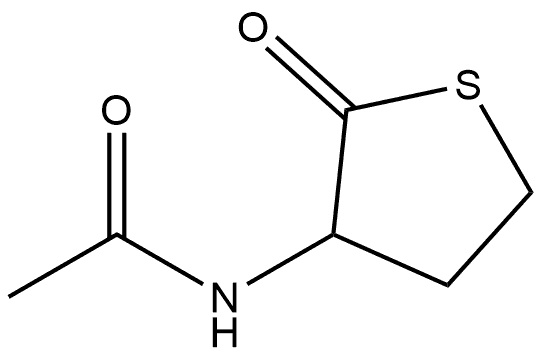
Acetamide, N-(tetrahydro-2-oxo-3-thienyl)-, (+)- synthesis
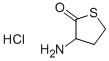
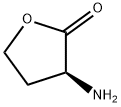
- Product Name:Acetamide, N-(tetrahydro-2-oxo-3-thienyl)-, (+)-
- CAS Number:768351-04-8
- Molecular formula:C6H9NO2S
- Molecular Weight:159.21
Yield:768351-04-8 97.1%
Reaction Conditions:
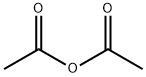
with sodium hydrogencarbonate in acetone at 57; under 760.051 Torr; for 3 h;Reagent/catalyst;Solvent;
Steps:
1
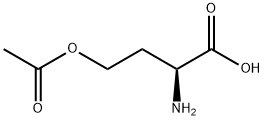
In normal pressure, at the reflux temperature of acetone (57 ), 3 hours ingredients in the flask, and stirred at 140 rpm, and reacted. After completion of the reaction, it was filtered DL- homocysteine thiolactone hydrochloride unreacted filter having a pore diameter of 0.2 μm. The filtrate of acetone and by-produced acetic acid to give the N- acetyl homocysteine thiolactone of 7.84g as a white solid by removal under reduced pressure. The results are shown in Table 2.
References:
JP2015/40175,2015,A Location in patent:Paragraph 0037; 0038
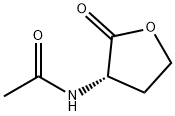
51524-71-1
27 suppliers
$64.00/5mg

768351-04-8
0 suppliers
inquiry