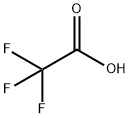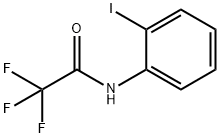
AcetaMide, 2,2,2-trifluoro-N-(2-iodophenyl)- synthesis
- Product Name:AcetaMide, 2,2,2-trifluoro-N-(2-iodophenyl)-
- CAS Number:143321-89-5
- Molecular formula:C8H5F3INO
- Molecular Weight:315.03
Yield:143321-89-5 100%
Reaction Conditions:
with triethylamine in tetrahydrofuran at -15 - 25; for 13 h;
Steps:
2-(3-Hydroxypropyl)indole (S7)32
To a solution of 2-iodoaniline (0.3 g, 1.37 mmol) and Et3N (0.22 mL, 1.64 mmol) in THF (3.4 mL) at -15°C was added dropwise a THF solution (1.0 mL) of (CF3CO)2O (0.2 mL, 1.51 mmol). The mixture was stirred at -15°C for 1 h, allowed to warm up to room temperature, and stirred for 10 h. The mixture was poured into H2O and extracted into Et2O (3 × 10 mL). The combined organic layer was dried over MgSO4, concentrated under reduced pressure, and chroatographed on a silica gel column (EtOAc/hexane = 1:2) to afford the acylation product S5 (0.41 g, quantative yield). Under an atmosphere of argon, a mixture of the above-prepared acylation compound S5 (0.41 g, 1.4 mmol), 3-butyn-1-ol (0.14 g, 1.64 mmol), Pd(OAc)2 (5.4 mg, 6.1 mol %), and Bu4NOAc (1.03 g, 3.41 mmol) in acetonitrile (17 mL) was heated at reflux (85°C) for 12 h. After completion of the reaction, the mixture was diluted with water, and extracted with EtOAc. The organic layer was separated, dried over MgSO4, and concentrated under reduced pressure. The residue was purified by silica gel flash column chromatography (CH2Cl2/MeOH = 20:1) to afford indole compound S7 (0.13 g, 55%).
References:
Hong, Bei-Tao;Chen, Chun-Lin;Fang, Jim-Min;Tsai, Keng-Chang;Wang, Shi-Yun;Huang, Wen-I;Cheng, Yih-Shyun E.;Wong, Chi-Huey [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 23,p. 6647 - 6654] Location in patent:supporting information
38222-83-2
245 suppliers
$6.00/250mg
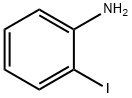
615-43-0
414 suppliers
$5.00/5g

143321-89-5
9 suppliers
inquiry