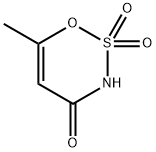
Acesulfame synthesis
- Product Name:Acesulfame
- CAS Number:33665-90-6
- Molecular formula:C4H5NO4S
- Molecular Weight:163.15
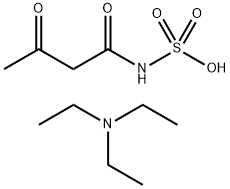
99911-48-5
0 suppliers
inquiry

33665-90-6
232 suppliers
inquiry
Yield:33665-90-6 37 - 91 %Chromat.
Reaction Conditions:
Stage #1: Triethylammoniumsalz der Acetoacetamid-N-sulfonsaeurewith disulfuric acid in dichloromethane at -30 - 4; for 0.00116667 - 0.00569444 h;
Stage #2: with water in dichloromethane at 0 - 35;Product distribution / selectivity;
Steps:
1-8
A reaction was carried out using, as the reactor, a stainless steel tube having an inner diameter of 2 mm and an effective length of 1 m. A total of 55 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 112 g of dichloromethane and was cooled to 4 C. Separately, 324 mmol of sulfuric anhydride was dissolved in 278 g of dichloromethane and was cooled to 4 C. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously fed at rates of 4.2 ml/min and 6.4 ml/min, respectively, into the reactor immersed in a refrigerant at ?30 C. The residence time was 18 seconds. The reaction mixture was continuously sampled from the reactor, was introduced into 120 g of water which had been cooled to 0 C. in an Erlenmeyer flask and was subjected to hydrolysis at temperatures of 0 C. to 10 C. with stirring using a magnetic stirrer. After the completion of the reaction, the reaction mixture was separated into a dichloromethane layer and an aqueous layer, and the aqueous layer was further extracted with two portions of 100 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 75% on the basis of triethylammonium acetoacetamide-N-sulfonate. EXAMPLE 2 A reaction was carried out using, as the reactor, a stainless steel tube having an inner diameter of 2 mm and an effective length of 1 m and equipped with a Kenics static mixer as a mixer for raw materials (premixer). The Kenics static mixer had an inner diameter of 3.4 mm and a length of 10 cm and contained 17 elements. A total of 55 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 314 g of dichloromethane and was cooled to 4 C. Separately, 317 mmol of sulfuric anhydride was dissolved in 575 g of dichloromethane and was cooled to 4 C. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously fed at rates of 5.1 ml/min and 7.0 ml/min, respectively, into the premixer arranged at an inlet of the reactor immersed in a refrigerant at ?30 C. The residence times in the premixer and in the reactor were 4.5 seconds and 16 seconds, respectively. The reaction mixture was continuously sampled from the reactor, was introduced into 120 g of water which had been cooled to 0 C. in an Erlenmeyer flask and was subjected to hydrolysis at temperatures of 0 C. to 10 C. with stirring using a magnetic stirrer. After the completion of the reaction, the reaction mixture was separated into a dichloromethane layer and an aqueous layer, and the aqueous layer was further extracted with two portions of 300 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 89% on the basis of triethylammonium acetoacetamide-N-sulfonate. EXAMPLE 3 A reaction was carried out using, as the reactor, a Kenics static mixer having an inner diameter of 3.4 mm and a length of 10 cm and containing 17 elements. A total of 28 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 273 g of dichloromethane and was cooled to 4 C. Separately, 157 mmol of sulfuric anhydride was dissolved in 439 g of dichloromethane and was cooled to 4 C. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously fed at rates of 5.1 ml/min and 7.4 ml/min, respectively, into the reactor immersed in a refrigerant at ?30 C. The residence time was 4.3 seconds. The reaction mixture was continuously sampled from the reactor, was introduced into 120 g of water which had been cooled to 0 C. in an Erlenmeyer flask and was subjected to hydrolysis at temperatures of 0 C. to 10 C. with stirring using a magnetic stirrer. After the completion of the reaction, the reaction mixture was separated into a dichloromethane layer and an aqueous layer, and the aqueous layer was further extracted with two portions of 300 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 84% on the basis of triethylammonium acetoacetamide-N-sulfonate. EXAMPLE 4 A reaction was carried out using, as the reactor, a Kenics static mixer having an inner diameter of 3.4 mm and a length of 10 cm and containing 17 elements. A total of 28 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 362 g of dichloromethane and was cooled to 4 C. Separately, 139 mmol of sulfuric anhydride was dissolved in 585 g of dichloromethane and was cooled to 4 C. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously fed at rates of 5.5 ml/min and 7.4 ml/min, respectively, into the reactor immersed in a refrigerant at ?30 C. The residence time was 4.2 seconds. The reaction mixture was continuously sampled from the reactor, was introduced into 120 g of water which had been cooled to 0 C. in an Erlenmeyer flask and was subjected to hydrolysis at temperatures of 0 C. to 10 C. with stirring using a magnetic stirrer. After the completion of the reaction, the reaction mixture was separated into a dichloromethane layer and an aqueous layer, and the aqueous layer was further extracted with two portions of 300 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 86% on the basis of triethylammonium acetoacetamide-N-sulfonate. EXAMPLE 5 A reaction was carried out using, as the reactor, a Kenics static mixer having an inner diameter of 8.0 mm and a length of 26 cm and containing 17 elements. A total of 19 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 250 g of dichloromethane and was cooled to 3 C. Separately, 109 mmol of sulfuric anhydride was dissolved in 404 g of dichloromethane and was cooled to 3 C. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously fed at rates of 71 ml/min and 103 ml/min, respectively, into the reactor immersed in a refrigerant at ?30 C. The residence time was 4.5 seconds. The reaction mixture was continuously sampled from the reactor, was introduced into 120 g of water which had been cooled to 0 C. in an Erlenmeyer flask and was subjected to hydrolysis at temperatures of 0 C. to 10 C. with stirring using a magnetic stirrer. After the completion of the reaction, the reaction mixture was separated into a dichloromethane layer and an aqueous layer, and the aqueous layer was further extracted with two portions of 300 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 85% on the basis of triethylammonium acetoacetamide-N-sulfonate. EXAMPLE 6 A reaction was carried out using, as the reactor, a stainless steel tube having an inner diameter of 0.5 mm and an effective length of 2 m. A total of 28 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 363 g of dichloromethane and was cooled to 4 C. Separately, 157 mmol of sulfuric anhydride was dissolved in 583 g of dichloromethane and was cooled to 4 C. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously fed at rates of 2.1 ml/min and 3.2 ml/min, respectively, into the reactor immersed in a refrigerant at ?30 C. The residence time was 4.4 seconds. The reaction mixture was continuously sampled from the reactor, was introduced into 120 g of water which had been cooled to 0 C. in an Erlenmeyer flask and was subjected to hydrolysis at temperatures of 0 C. to 10 C. with stirring using a magnetic stirrer. After the completion of the reaction, the reaction mixture was separated into a dichloromethane layer and an aqueous layer, and the aqueous layer was further extracted with two portions of 300 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 87% on the basis of triethylammonium acetoacetamide-N-sulfonate. EXAMPLE 7 A reaction was carried out using, as the reactor, a stainless steel tube having an inner diameter of 4 mm and an effective length of 2 m. The reactor was equipped with a Kenics static mixer having an inner diameter of 8.0 mm and a length of 26 cm and containing 17 elements as a mixer for raw materials (premixer), had an insert tube at piping joint of a raw material mixing section, in which two liquids start to be mixed in the static mixer section. A total of 60 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 300 g of dichloromethane and was cooled to ?10 C. Separately, 382 mmol of sulfuric anhydride was dissolved in 418 g of dichloromethane and was cooled to ?10 C. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously fed at rates of 141 ml/min and 199 ml/min, respectively, into the premixer arranged at an inlet of the reactor immersed in a refrigerant at ?30 C. The residence times in the premixer and in the reactor were 2.3 seconds and 4.4 seconds, respectively. The reaction mixture was continuously sampled from the reactor, was introduced into 60 g of water in an Erlenmeyer flask and was subjected to hydrolysis at temperatures of 15 C. to 25 C. with stirring using a magnetic stirrer. After the completion of the reaction, the reaction mixture was separated into a dichloromethane layer and an aqueous layer, and the aqueous layer was further extracted with two portions of 200 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 91% on the basis of triethylammonium acetoacetamide-N-sulfonate. EXAMPLE 8 A reaction was carried out using, as the reactor, a stainless steel tube having an inner diameter of 4 mm and an effective length of 2 m. This reactor was equipped with a Kenics static mixer having an inner diameter of 8.0 mm and a length of 26 cm and containing 17 elements as a mixer for raw materials (premixer) and had an insert tube at piping joint of a raw material mixing section, in which two liquids start to be mixed in the static mixer section. A total of 134 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 400 g of dichloromethane and was cooled to ?10 C. Separately, 950 mmol of sulfuric anhydride was dissolved in 533 g of dichloromethane and was cooled to ?10 C. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously fed at rates of 139 ml/min and 193 ml/min, respectively, into the premixer arranged at an inlet of the reactor immersed in a refrigerant at ?30 C. The residence times in the premixer and in the reactor were 2.4 seconds and 4.5 seconds, respectively. The reaction mixture was continuously sampled from the reactor, was introduced into 60 g of water in an Erlenmeyer flask and was subjected to hydrolysis at temperatures of 15 C. to 35 C. with stirring using a magnetic stirrer. After the completion of the reaction, the reaction mixture was separated into a dichloromethane layer and an aqueous layer, and the aqueous layer was further extracted with two portions of 200 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 85% on the basis of triethylammonium acetoacetamide-N-sulfonate. COMPARATIVE EXAMPLE 1 A glass WFE having an inner diameter of 5 cm and a length of 20 cm and equipped with three wipers was used as the reactor. A total of 40 mmol of triethylammonium acetoacetamide-N-sulfonate was dissolved in 60 g of dichloromethane and was cooled to 0 C. Separately, 229 mmol of sulfuric anhydride was dissolved in 94 g of dichloromethane and was cooled to 0 C. A refrigerant at ?30 C. was allowed to flow into the jacket of the reactor, and the wipers were rotated at a rate of 700 rpm. The triethylammonium acetoacetamide-N-sulfonate solution and the sulfuric anhydride solution were continuously added dropwise to the stirring axis of the wipers and to the wall of the WFE reactor, respectively, over sixteen minutes for reaction. The reaction mixture discharged from the bottom of the WFE reactor was continuously fed to 20 g of water in a flask cooled to 0 C. on an ice bath, and the mixture was subjected to hydrolysis at 0 C. to 10 C. with stirring using a magnetic stirrer. After the completion of the reaction, 100 g of dichloromethane and 10 g of water were added, the mixture was stirred and was separated into a dichloromethane layer and an aqueous layer. The aqueous layer was further extracted with two portions of 100 ml of dichloromethane. The amount of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one-2,2-dioxide in the combined dichloromethane layers was determined by HPLC to find that the yield was 37% on the basis of triethylammonium acetoacetamide-N-sulfonate.
References:
US2005/182255,2005,A1 Location in patent:Page/Page column 5-7
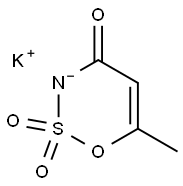
55589-62-3
402 suppliers
$7.00/25g

33665-90-6
232 suppliers
inquiry
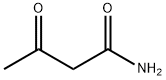
5977-14-0
180 suppliers
$48.00/100g
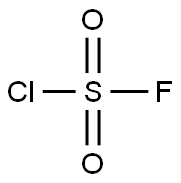
13637-84-8
26 suppliers
$685.00/25g

33665-90-6
232 suppliers
inquiry