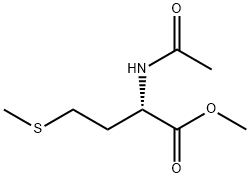
AC-MET-OME synthesis
- Product Name:AC-MET-OME
- CAS Number:35671-83-1
- Molecular formula:C8H15NO3S
- Molecular Weight:205.27
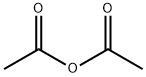
108-24-7
0 suppliers
$14.00/250ML
2491-18-1
283 suppliers
$6.00/5g

35671-83-1
73 suppliers
$50.00/100mg
Yield:35671-83-1 94%
Reaction Conditions:
with pyridine at 20;Cooling with ice;
Steps:
4.2.1. N-Ac-l-Met-OMe
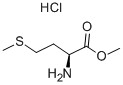
In a typical reaction, 73 mL of acetic anhydride (780 mmol) and 63 mL pyridine (780 mmol) were combined in a round-bottomed flask and chilled on ice. After 5-10 min, 8.6 g (100 mmol) l-methionine methyl ester HCl were added and the reaction mixture was allowed to slowly return to room temperature overnight. The next morning, the reaction was quenched with cold water and extracted four times with 75 mL of methylene chloride. The extracts were then rinsed three times each with 1 M HCl, saturated sodium bicarbonate solution, and water. The extracts were then dried over MgSO4, filtered, and evaporated under reduced pressure, yielding a yellow oil which crystallized upon standing. The crude product was recrystallized in ethyl ether at -20 °C. Crystals (19.3 g, 94 mmol, 94%) were isolated by vacuum filtration. Mp = 41.7-42.4 °C. 1H NMR ([2H]-chloroform, TMS = 0.0 ppm): δ 1.86-2.20 (m, 2H), δ 2.00 (s, 3H), δ 2.05 (s, 3H), δ 2.45-2.60 (m, 2H), δ 3.75 (s, 3H), δ 4.62-4.70 (m, 1H), δ 6.13-6.19 (bd, 1H).
References:
Foster, Michael S.;Oldham, Charlie D.;May, Sheldon W. [Tetrahedron Asymmetry,2011,vol. 22,# 3,p. 283 - 293] Location in patent:experimental part
67-56-1
776 suppliers
$9.00/25ml
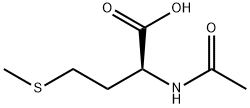
65-82-7
358 suppliers
$6.00/5g

35671-83-1
73 suppliers
$50.00/100mg
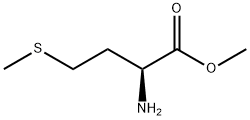
10332-17-9
21 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML

35671-83-1
73 suppliers
$50.00/100mg
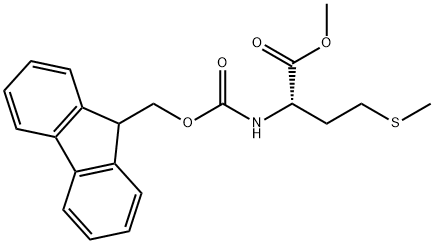
500872-34-4
2 suppliers
inquiry

35671-83-1
73 suppliers
$50.00/100mg
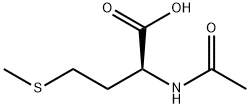
1115-47-5
416 suppliers
$6.00/25g

35671-83-1
73 suppliers
$50.00/100mg
