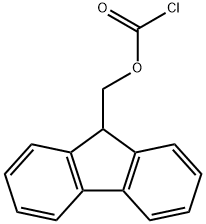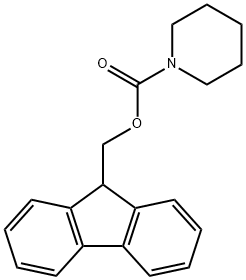
(9H-fluoren-9-yl)methyl piperidine-1-carboxylate synthesis
- Product Name:(9H-fluoren-9-yl)methyl piperidine-1-carboxylate
- CAS Number:207558-19-8
- Molecular formula:C20H21NO2
- Molecular Weight:307.39
Yield:207558-19-8 77%
Reaction Conditions:
in neat (no solvent) at 20; for 0.0833333 h;Sonication;Irradiation;Green chemistry;
Steps:
General procedure of N-Fmoc protection on amines derivatives promoted by ultrasound irradiations
General procedure: Amine (1 mmol) and Fmoc-Cl (1.1 mmol) were placed in a glass tube under neat conditions and were sonicated for a suitable time (as indicated in Tables 1, 2 and 3). All reactions were performed in a water bath at room temperature. After completion of the reaction (as indicated by TLC), 5 cm3 of diethyl ether was added to the mixture. The N-Fmoc derivatives were crystallized and were obtained in good to excellent yields. Purification of the product was accomplished by recrystallization from diethyl ether.
References:
Mansouri, Rachida;Aouf, Zineb;Lakrout, Salah;Berredjem, Malika;Aouf, Nour-Eddine [Journal of the Brazilian Chemical Society,2016,vol. 27,# 3,p. 546 - 550]
209115-33-3
39 suppliers
$45.00/50mg

207558-19-8
9 suppliers
inquiry
35661-40-6
419 suppliers
$5.00/5g

207558-19-8
9 suppliers
inquiry
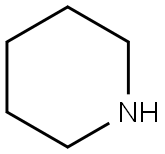
110-89-4
0 suppliers
$15.96/100ML
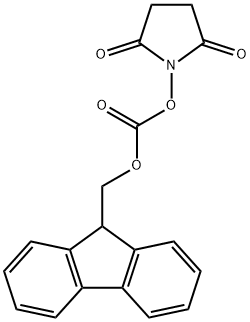
82911-69-1
645 suppliers
$7.00/25g

207558-19-8
9 suppliers
inquiry