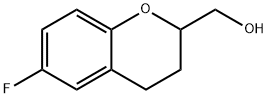
rac 6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol synthesis
- Product Name:rac 6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol
- CAS Number:99199-62-9
- Molecular formula:C10H11FO2
- Molecular Weight:182.19
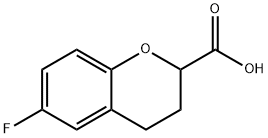
99199-60-7
233 suppliers
$70.68/5gm:

99199-62-9
21 suppliers
$5819.00/10g
Yield:99199-62-9 92%
Reaction Conditions:
Stage #1: 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acidwith sodium tetrahydroborate;sulfuric acid in tetrahydrofuran;diethyl ether at 0 - 35; for 0.75 h;
Stage #2: with sodium hydroxide in water; pH=7;Product distribution / selectivity;
Steps:
1 Preparation of (6-fluorochroman-2-yl)methanol (Formula VIII)
EXAMPLE 1 Preparation of 6-(fluorochroman-2-yl)methanol (Formula VIII) A round bottom flask is charged with 6-fluorochroman-2-carboxylic acid (100 g) and tetrahydrofuran (1 L) and the mixture is cooled to 0° C. Sodium borohydride (48.19 g) is slowly added at 0-5° C., then a solution of conc. sulfuric acid (33.95 mL) in ether (68 mL) is added by drops at 0-5° C. and the mixture is stirred at 35° C. for 30-45 minutes. Reaction completion is verified using thin layer chromatography (TLC), and the reaction mixture is cooled to 0° C. followed by quenching of the reaction by slow addition of methanol (300 mL). The solvent is distilled completely under reduced pressure, water (500 mL) is added to the residue, and pH of the mixture is adjusted to 7 by addition by drops of sodium hydroxide solution. The mass is extracted with dichloromethane (1000 mL, then 500 mL), followed by washing of the combined organic layer with water (500 mL) and brine solution (500 mL) and drying with sodium sulfate (50 g). The solvent is distilled under reduced pressure to afford the title compound (85 g, 92% yield).
References:
US2011/250454,2011,A1 Location in patent:Page/Page column 12
874649-82-8
88 suppliers
$9.00/250mg
409346-73-2
16 suppliers
inquiry

99199-62-9
21 suppliers
$5819.00/10g
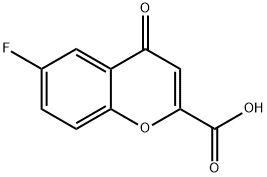
99199-59-4
143 suppliers
$34.46/1g

99199-62-9
21 suppliers
$5819.00/10g

99199-60-7
233 suppliers
$70.68/5gm:

409346-73-2
16 suppliers
inquiry

99199-62-9
21 suppliers
$5819.00/10g