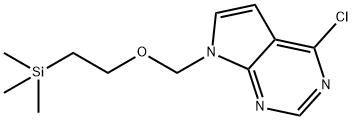
4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE synthesis
- Product Name:4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE
- CAS Number:941685-26-3
- Molecular formula:C12H18ClN3OSi
- Molecular Weight:283.83
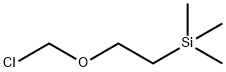
76513-69-4
362 suppliers
$6.00/1g
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
845 suppliers
$6.00/1g
![4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/941685-26-3.gif)
941685-26-3
185 suppliers
$59.00/250mg
Yield:941685-26-3 100%
Reaction Conditions:
Stage #1: 4-chloro-1H-pyrrolo[2,3-d]pyrimidinewith sodium hydride in N,N-dimethyl-formamide at 20; for 1 h;Cooling with ice;
Stage #2: (2-trimethylethylsilylethoxy)methyl chloride in N,N-dimethyl-formamide;Cooling with ice;
Steps:
1.D Step D: 4-chloro-7-(2-(trimethylsilyl)ethoxy)methyl-7H-pyrrolo[2,3-d]pyrimidine
Under ice-cooling with stirring, 38.4g (250.4mmol, 1.0eq.) 4-chloro-7H-pyrrolo[2,3-d]pyrimidine is dissolved in 200mL dry DMF. 13g (305mmol, 1.2eq) 57% content of NaH was added. The reaction was stirred at room temperature for 1 hour. 50.9g SEMCl was added dropwise under ice-cooling (305mmol, 1.2eq.). After complete addition, the ice bath the reaction was stirred for 1 hour, quenched with water, extracted with ethyl acetate, combined organic phases were washed with brine, dried over sodium sulfate, filtered and concentrated in vacuo, column chromatography on a silica column to give the title compound (71g , yield = 100%).
References:
CN109867676,2019,A Location in patent:Paragraph 0109-0111
![Pyrrolo[2,3-d]pyrimidin-4-ol](/CAS/GIF/3680-71-5.gif)
3680-71-5
411 suppliers
$6.00/1g
![4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/941685-26-3.gif)
941685-26-3
185 suppliers
$59.00/250mg