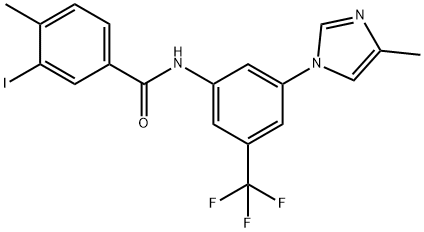
3-Iodo-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]benzamide synthesis
- Product Name:3-Iodo-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]benzamide
- CAS Number:926922-18-1
- Molecular formula:C19H15F3IN3O
- Molecular Weight:485.24
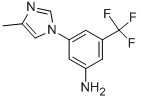
641571-11-1
343 suppliers
$9.00/1g
82998-57-0
241 suppliers
$5.00/1g
![3-Iodo-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]benzamide](/CAS2/GIF/926922-18-1.gif)
926922-18-1
34 suppliers
$120.00/50mg
Yield:926922-18-1 100%
Reaction Conditions:
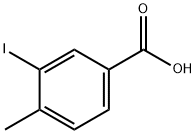
Stage #1: 3-Iodo-4-methyl-benzoic acidwith thionyl chloride for 2 h;Reflux;
Stage #2: 5-(4-methyl-1H-imidazol-1-yl)-3-(trifluoromethyl)-benzenaminewith dmap;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 20; for 17 h;
Steps:
1.1 Step 1: Preparation of 3-iodo-4-methyl-N-[3-(4-methyl-lH-imidazol-l-yl)-5- (trifluoromethy l)pheny 1] benzamide
A solution of 3-iodo-4-methylbenzoic acid (7.00 g, 26.7 mmol) in SOCh (47 mL) was refluxed for 2 h, then evaporated in vacuo to remove residual SOCI2. The residue was dissolved in anhydrous THF (25 mL) and added dropwise to a solution of DIPEA (4.14 g, 32.0 mmol), 3-(4-methyl-lH-imidazol-l-yl)-5-(trifluoromethyl)aniline (6.44 g, 26.7 mmol) and DMAP (130 mg, 1.06 mmol) in anhydrous THF (48 mL). The reaction mixture was stirred at RT for 17 h and evaporated in vacuo. The residue was dissolved in EtOAc (200 mL). Water was added (180 mL) and the pH was adjusted to 8 with 1 M NaOH. The layers were then separated and aqueous layer was extracted with DCM/MeOH 100:5 (100 mL x5). The combined organic extracts were evaporated in vacuo to give the final product as off-white solid (13.05 g, 100%). 10.67 (s, 1H), 8.46 (d, 7 = 1.9 Hz, 1H), 8.27 (t, 7 = 1.9 Hz, 1H), 8.21 (d, 7 = 1.4 Hz, 1H), 8.13 (d, 7 = 1.8 Hz, 1H), 7.94 (dd, 7 = 7.9, 1.9 Hz, 1H), 7.74 (d, 7 = 1.8 Hz, 1H), 7.53 (d, 7= 8.0 Hz, 1H), 7.49 (d, 7= 1.6 Hz, 1H), 2.46 (s, 3H), 2.18 (d, 7 = 1.0 Hz, 3H).
References:
WO2021/239643,2021,A1 Location in patent:Page/Page column 63-64

641571-11-1
343 suppliers
$9.00/1g
52107-98-9
28 suppliers
$236.81/1gm:
![3-Iodo-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]benzamide](/CAS2/GIF/926922-18-1.gif)
926922-18-1
34 suppliers
$120.00/50mg

641571-11-1
343 suppliers
$9.00/1g
![3-Iodo-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]benzamide](/CAS2/GIF/926922-18-1.gif)
926922-18-1
34 suppliers
$120.00/50mg

82998-57-0
241 suppliers
$5.00/1g
![3-Iodo-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]benzamide](/CAS2/GIF/926922-18-1.gif)
926922-18-1
34 suppliers
$120.00/50mg
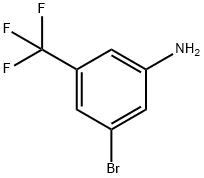
54962-75-3
302 suppliers
$5.00/1g
![3-Iodo-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]benzamide](/CAS2/GIF/926922-18-1.gif)
926922-18-1
34 suppliers
$120.00/50mg