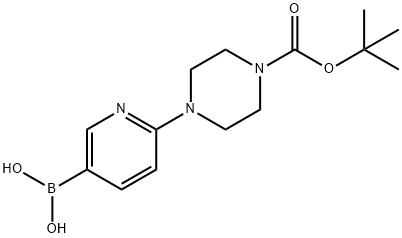
6-(4-N-BOC-PIPERAZINE-1-YL)-3-PYRIDINYL BORONIC ACID synthesis
- Product Name:6-(4-N-BOC-PIPERAZINE-1-YL)-3-PYRIDINYL BORONIC ACID
- CAS Number:919347-67-4
- Molecular formula:C14H22BN3O4
- Molecular Weight:307.15
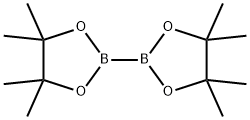
73183-34-3
581 suppliers
$6.00/5g
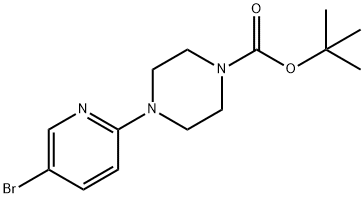
153747-97-8
95 suppliers
$7.00/250mg

919347-67-4
42 suppliers
$110.00/1g
![4-[5-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-PYRIDIN-2-YL]-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER](/CAS/GIF/496786-98-2.gif)
496786-98-2
134 suppliers
$6.00/250mg
Yield:-
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 100; for 20.25 h;Inert atmosphere;
Steps:
II Step II: tert-butyl 4- [5- (4, 4, 5, 5-tetramethyl-l, 3, 2- dioxaborolan-2-yl) -2-pyridyl] piperazine-l-carboxylate ( Intermediate 1-XIII)
A mixture of 1-11 (4.30 g, 12.57 mmol) , bis (pinacolato) diboron (3.82 g 15.07 mmol) and KOAc (2.94 g, 37.69 mmol) in 1,4-dioxane (30 mL) was degassed in a stream of argon for 15 minutes. To the mixture was added 1,1- bis (diphenylphosphino) ferrocene-palladium(II) dichloride dichloromethane complex (0.307 g, 0.376 mmol), and the reaction mixture was again degassed for additional 15 minutes. After stirring at 100°C for 20 hours, the volatiles were removed by evaporation, and the obtained residue was diluted with water (50 mL) , followed by extraction with ethyl acetate (50 mL x 3) . The combined organic layers were washed with brine (50 mL) , dried over anhydrous Na2SC> and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (100-200 mesh) using 50% EtOAc in hexanes to give the desired product Intermediate 1- XIII as a mixture of minor boronate ester together with major boronic acid . (4.8 g, crude yield 98%) as a yellow solid; LCMS (for boronate ester) : m/z 390.2 [M+l]; LCMS (for boronic acid) : m/z 308.1 [M+l] .
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED;KOUL, Summon;KURHADE, Suresh;BHOSALE, Sandeep;NAIK, Keshav;SALUNKHE, Videsh;MUNOT, Yogesh;BHUNIYA, Debnath WO2015/88045, 2015, A1 Location in patent:Paragraph 0239