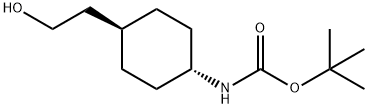
CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester synthesis
- Product Name:CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester
- CAS Number:917342-29-1
- Molecular formula:C13H25NO3
- Molecular Weight:243.34
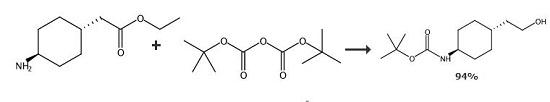
An 500 ml four-necked flask is charged with 40 g (0,18 mol) of trans 2-[1-(4-amino]- cyclohexyl)-acetic acid ethyl ester hydrochloride and 160 ml of dichloromethane. To the resulting suspension 18,2 g (0,18 mol) of triethylamine is added. The reaction mixture is cooled to a temperature between 8-10°C and a solution of 40,0 g (0,185 mol) of di(tert-butyl)dicarbonate in 100 ml of dichloromethane is added for 1 hour with stirring under nitrogen. Then the reaction mixture is allowed to warm to a temperature between 22-25°C and stirred until the reaction proceeds. After completion of the reaction 100 g of 5% aqueous sodium carbonate is added and the phases are separated. The organic layer is extracted with 50 ml of water and after separation the organic layer is dried under Na2SO4 and the filtrate is concentrated in vacuum. The trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-acetic acid ethyl ester obtained is dissolved in 460 ml of tetrahydrofurane then 13,68 g (0,36 mol) of sodium borohydride is added at 25°C under nitrogen. With stirring, to the reaction mixture a solution of 24,0 g (0,18 mol) of aluminium chloride in 250 ml abs. tetrahydrofurane is added dropwise at a temperature between 18-22°C for 1 hour under nitrogen then the mixture is stirred for additional 2 hours. After completion of the reaction the mixture is cooled to a temperature between 5-10°C and 650 ml of water and 600 ml of toluene are added. Then the pH was adjusted to 3-4 by adding 40-45 ml of concentrated hydrochloric acid and the stirring was continued at a temperature between 20-25°C for 1 hour. The phases are separated, the aqueous layer is extracted with 50 ml of toluene and the combined organic layers are washed with 3×150 ml of water and dried in vacuum. In this manner 41,1 g of title compound was obtained. Yield: 94% 20. Melting point: 101-103°C.
![Ethyl trans-2-[4-(Boc-aMino)cyclohexyl]acetate](/CAS/GIF/946598-34-1.gif)
946598-34-1
72 suppliers
$51.00/250mg
![CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester](/CAS/GIF/917342-29-1.gif)
917342-29-1
103 suppliers
$39.00/100mg
Yield:917342-29-1 95%
Reaction Conditions:
Stage #1: ethyl 2-(trans-4-((tert-butoxycarbonyl)amino)cyclohexyl)acetatewith lithium aluminium hydride in tetrahydrofuran at -5 - 0;
Stage #2: with sodium hydroxide in tetrahydrofuran;water monomer; for 0.5 h;Temperature;
Steps:
7 Trans 2- {1- [4- (N-tert-butoxycarbonyl) -amino] -cyclohexyl} ethanol
Ethyl 2- {1- [4- (N-tert-butoxycarbonyl) amino] cyclohexyl} acetate (50 g) and THF (300 ml) were added to a 1000 ml four-neck reaction flask,Cooling to 0 ,A mixture of lithium aluminum tetrahydrate (9 g) and THF (300 ml) was added dropwise,-5 ° C for 1-2 hours; after completion of the reaction,Water (9 g) was added dropwise to the reaction solution,30% aqueous sodium hydroxide solution (9 g) and water (27 g)Stir for 30 minutes.filter,The filtrate was added with 300 ml of water and 300 ml of toluene to separate the extract;The aqueous phase was extracted with 200 ml of toluene,Combine organic phase,Washed with saturated brine (300 ml x 2)Dried over anhydrous magnesium sulfate.The resulting white solid was filtered and concentrated,Recrystallization from acetonitrile,Dried at 50 ° C under vacuum to give 40.5 g of a white solid,Yield 95%.
References:
CN106543039,2017,A Location in patent:Paragraph 0076-0079
215789-45-0
18 suppliers
$120.00/10mg
![CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester](/CAS/GIF/917342-29-1.gif)
917342-29-1
103 suppliers
$39.00/100mg
179321-49-4
312 suppliers
$5.00/1g
![CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester](/CAS/GIF/917342-29-1.gif)
917342-29-1
103 suppliers
$39.00/100mg
![Carbamic acid, N-[trans-4-(cyanomethyl)cyclohexyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1313279-47-8.gif)
1313279-47-8
32 suppliers
inquiry
![CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester](/CAS/GIF/917342-29-1.gif)
917342-29-1
103 suppliers
$39.00/100mg