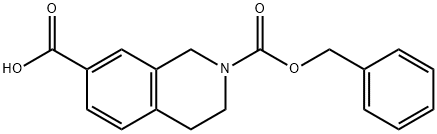
2-((benzyloxy)carbonyl)-1,2,3,4-tetrahydroisoquinoline-7-carboxylic acid synthesis
- Product Name:2-((benzyloxy)carbonyl)-1,2,3,4-tetrahydroisoquinoline-7-carboxylic acid
- CAS Number:877861-35-3
- Molecular formula:C18H17NO4
- Molecular Weight:311.33
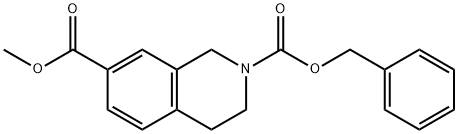
1187304-45-5
0 suppliers
inquiry

877861-35-3
14 suppliers
inquiry
Yield:877861-35-3 96%
Reaction Conditions:
with water;lithium hydroxide in methanol; for 2 h;
Steps:
67.2 Step 2. Synthesis of 2-((benzyloxy)carbonyl)-1,2,3,4-tetrahydroisoquinoline-7-carboxylic acid .
Asolution of LiOH (0.77 g, 18.3 mmol, 5.0 eq) in H2O (9.8 mL) was added to solution of 2-benzyl 7-methyl 3,4-dihydroisoquinoline-2,7(1H)-dicarboxylate (1.2 g, 3.7 mmol, 1.0 eq) in MeOH (14.6 mL) and the reaction was stirred for 2 hrs. The reaction was partitioned between MTBE and H2O. The layers were separated and the aqueous layer was adjusted to an acidic pH (~5) with 0.1M aqueous HCl. The resulting mixture was extracted with EtOAc (2 ). The combined organic layers were dried (MgSO4), filtered, and concentrated under reduced pressure to afford 2-((benzyloxy)carbonyl)-1,2,3,4- tetrahydroisoquinoline-7-carboxylic acid (1.1 g, 3.5 mmol, 96%) as a pale yellow solid: 1H NMR (400 MHz, DMSO-d6) δ 7.77 (d, J = 1.7 Hz, 1H), 7.73 (dd, J = 7.9, 1.7 Hz, 1H), 7.42- 7.30 (comp, 4H), 7.29 (d, J = 8.0 Hz, 1H), 5.13 (s, 2H), 4.64 (d, J = 20.0 Hz, 2H), 3.65 (d, J = 6.7 Hz, 2H), 2.87 (t, J = 5.9 Hz, 2H).
References:
WO2018/218051,2018,A1 Location in patent:Page/Page column 71