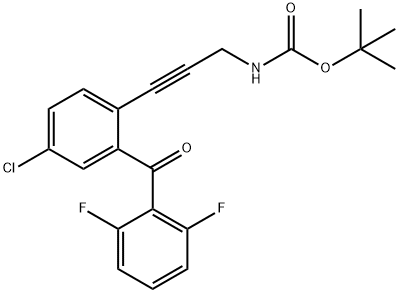
[3-[4-Chloro-2-(2,6-difluorobenzoyl)phenyl]prop-2-ynyl]carbamic acid tert-butyl ester synthesis
- Product Name:[3-[4-Chloro-2-(2,6-difluorobenzoyl)phenyl]prop-2-ynyl]carbamic acid tert-butyl ester
- CAS Number:869366-03-0
- Molecular formula:C21H18ClF2NO3
- Molecular Weight:405.82
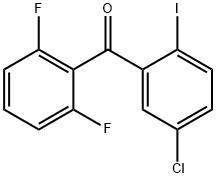
869365-97-9
55 suppliers
$75.00/100mg
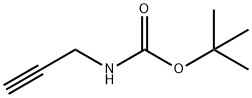
92136-39-5
209 suppliers
$6.00/1g
![[3-[4-Chloro-2-(2,6-difluorobenzoyl)phenyl]prop-2-ynyl]carbamic acid tert-butyl ester](/CAS/GIF/869366-03-0.gif)
869366-03-0
17 suppliers
$503.77/5MG
Yield:869366-03-0 61%
Reaction Conditions:
Stage #1: (5-chloro-2-iodophenyl)(2,6-difluorophenyl)methanone;N-tert-Butoxycarbonyl-1-amino-3-propyne;bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide in dichloromethane; for 0.5 h;
Stage #2: with diethylamine in dichloromethane at 20; for 16 h;
Steps:
8.I {3-[4-Chloro-2-(2,6-difluoro-benzoyl)-phenyl]-prop-2-ynyl}-carbamic acid tert-butyl ester (3aa)
{3-[4-Chloro-2-(2,6-difluoro-benzoyl)-phenyl]-prop-2-ynyl}-carbamic acid tert-butyl ester (3aa) (5-Chloro-2-iodo-phenyl)-(2,6-difluoro-phenyl)-methanone (2aa) (5.5 g, 14.5 mmol), prop-2-ynyl-carbamic acid tert-butyl ester (2.5 g, 16 mmol), PdCl2(PPh3)2 (0.6 g, 0.9 mmol) and Cu(I)I (0.2 g, 0.9 mmol) were suspended in anhydrous CH2Cl2 (50 mL) and the mixture was sparged with nitrogen for 30 min. Diethylamine (8 mL) was added and the solution was stirred at room temperature for 16 h. The solution was concentrated in vacuo and the resulting residue purified by column chromatography (silica gel, 0 to 15% EtOAc/hexanes) to afford 3aa (3.6 g, 61%) as a white solid, MS m/z=406 (M+H).
References:
US2005/256102,2005,A1 Location in patent:Page/Page column 142-143
28910-83-0
127 suppliers
$11.00/250mg
![[3-[4-Chloro-2-(2,6-difluorobenzoyl)phenyl]prop-2-ynyl]carbamic acid tert-butyl ester](/CAS/GIF/869366-03-0.gif)
869366-03-0
17 suppliers
$503.77/5MG
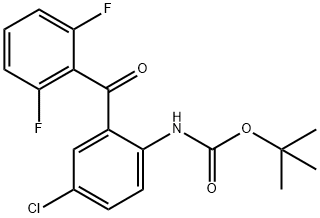
869365-92-4
3 suppliers
inquiry
![[3-[4-Chloro-2-(2,6-difluorobenzoyl)phenyl]prop-2-ynyl]carbamic acid tert-butyl ester](/CAS/GIF/869366-03-0.gif)
869366-03-0
17 suppliers
$503.77/5MG
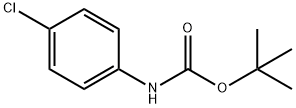
18437-66-6
77 suppliers
$11.00/5g
![[3-[4-Chloro-2-(2,6-difluorobenzoyl)phenyl]prop-2-ynyl]carbamic acid tert-butyl ester](/CAS/GIF/869366-03-0.gif)
869366-03-0
17 suppliers
$503.77/5MG
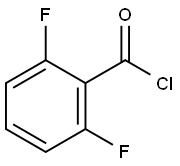
18063-02-0
257 suppliers
$12.00/5g
![[3-[4-Chloro-2-(2,6-difluorobenzoyl)phenyl]prop-2-ynyl]carbamic acid tert-butyl ester](/CAS/GIF/869366-03-0.gif)
869366-03-0
17 suppliers
$503.77/5MG