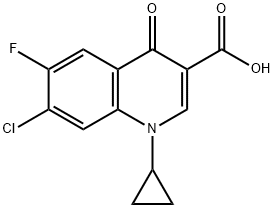
7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid synthesis
- Product Name:7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid
- CAS Number:86393-33-1
- Molecular formula:C13H9ClFNO3
- Molecular Weight:281.67
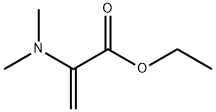
16173-58-3
0 suppliers
inquiry
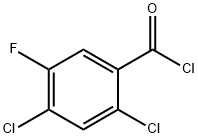
86393-34-2
182 suppliers
$7.00/5g
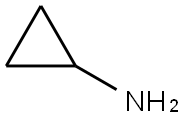
765-30-0
502 suppliers
$10.00/25g

86393-33-1
345 suppliers
$15.00/25g
Yield:86393-33-1 86%
Reaction Conditions:
with sodium hydroxide;potassium carbonate;acetic acid;triethylamine in 5,5-dimethyl-1,3-cyclohexadiene;water;isopropyl alcohol
Steps:
2 Example 2
Example 2 A mixture of 380 g of xylene (mixture of isomers), 110 g of ethyl N,N-dimethylaminoacrylate and 77.4 g of triethylamine was initially charged, and 160 g of 2,4-dichloro-5-fluorobenzoyl chloride were added dropwise at 70° C. over a period of 60 minutes. The mixture was subsequently stirred at 70° C. for 2 hours and cooled to room temperature. At room temperature, 51 g of acetic acid were then added, and the mixture was again heated to 70° C. At 70° C., 45 g of cyclopropylamine were then added dropwise. 100 ml of water were added to the reaction mixture which was stirred for 15 minutes, and the organic phase that formed was separated off. The organic phase was metered into a mixture of 89 g of potassium carbonate and 190 g of xylene (mixture of isomers) at from 140 to 142° C. The water of reaction that was liberated was separated off via a water separator. After all the water had been separated off, the mixture was cooled to 80° C. and, at a pressure of 40 mbar, xylene was distilled off. 80 g of 45% strength aqueous sodium hydroxide solution and 350 g of water were then added, and the remaining xylene was distilled off. After addition of 180 g of acetic acid and 100 g of water, the product was filtered off with suction and the solid was washed 3 times with 150 ml of water each time and 3 times with 200 ml of isopropanol each time. Drying under reduced pressure at 60° C. gave 170 g of 1-cyclopropyl-7-chloro-6-fluoro-1,4-dihydro-4-oxo-3-quinoline-carboxylic acid. This corresponds to a yield of 86% of theory.
References:
Bayer Aktiengesellschaft US6229017, 2001, B1
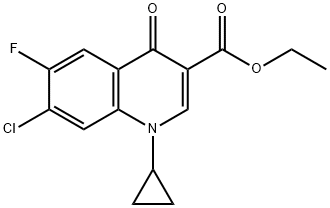
86483-54-7
9 suppliers
inquiry

86393-33-1
345 suppliers
$15.00/25g
![Benzenepropanoic acid, 2,4-dichloro-α-[(cyclopropylamino)methylene]-5-fluoro-β-oxo-, ethyl ester, (αZ)-](/CAS/20210305/GIF/104600-30-8.gif)
104600-30-8
0 suppliers
inquiry

86393-33-1
345 suppliers
$15.00/25g
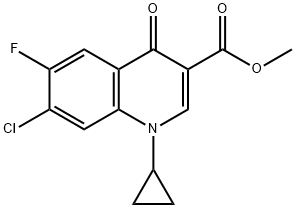
104599-90-8
9 suppliers
inquiry

86393-33-1
345 suppliers
$15.00/25g
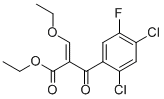
86483-52-5
6 suppliers
inquiry

765-30-0
502 suppliers
$10.00/25g

86393-33-1
345 suppliers
$15.00/25g