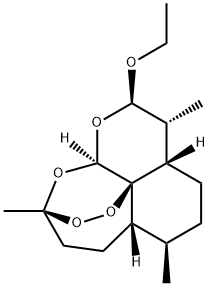
(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin synthesis
- Product Name:(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin
- CAS Number:82534-75-6
- Molecular formula:C17H28O5
- Molecular Weight:312.4

64-17-5
756 suppliers
$10.00/50g
71939-50-9
402 suppliers
$5.00/50mg
![(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin](/CAS/GIF/82534-75-6.gif)
82534-75-6
12 suppliers
inquiry
Yield:82534-75-6 55%
Reaction Conditions:
with boron trifluoride diethyl etherate in dichloromethane at 20; for 7 h;
Steps:
4.1.1 General procedure for the synthesis of compound 2
General procedure: To a solution of dihydroartemisinin (1, 1mmol, 1.0 equiv.) in 5mL DCM was added BF3-Et2O (0.2mmol, 12.6μL, 0.2 equiv.) and aliphatic alcohol (2mmol, 2 equiv.) slowly, the resulting mixture was stirred at room temperature for 7h until the reaction completed. The saturated of NaHCO3 was added, the mixture was further diluted with DCM, and washed with H2O and brine. The organic layer was dried by anhydrous Na2SO4, concentrated, and purified by column chromatography on silica gel to give the corresponding compound 2.
References:
Wang, Shuo;Wang, Hongshuang;Lin, Cong;Zhang, Tianshu;Gao, Jingwei;Wu, Siru;Wang, Yibo;Li, Hongyuan;Min, Weihong;Liu, Chunlei;Wang, Xiaohui [Bioorganic Chemistry,2021,vol. 114,art. no. 105107]

64-17-5
756 suppliers
$10.00/50g

71939-50-9
402 suppliers
$5.00/50mg
![(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin](/CAS/GIF/82534-75-6.gif)
82534-75-6
12 suppliers
inquiry

75887-54-6
191 suppliers
$35.00/1mg

64-17-5
756 suppliers
$10.00/50g

71939-50-9
402 suppliers
$5.00/50mg
![(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin](/CAS/GIF/82534-75-6.gif)
82534-75-6
12 suppliers
inquiry

75887-54-6
191 suppliers
$35.00/1mg
63968-64-9
642 suppliers
$5.00/250mg
![(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin](/CAS/GIF/82534-75-6.gif)
82534-75-6
12 suppliers
inquiry

64-17-5
756 suppliers
$10.00/50g

63968-64-9
642 suppliers
$5.00/250mg
![(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin](/CAS/GIF/82534-75-6.gif)
82534-75-6
12 suppliers
inquiry

75887-54-6
191 suppliers
$35.00/1mg