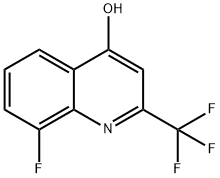
8-FLUORO-4-HYDROXY-2-(TRIFLUOROMETHYL)QUINOLINE synthesis
- Product Name:8-FLUORO-4-HYDROXY-2-(TRIFLUOROMETHYL)QUINOLINE
- CAS Number:31009-31-1
- Molecular formula:C10H5F4NO
- Molecular Weight:231.15
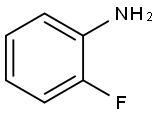
348-54-9
431 suppliers
$10.00/1g
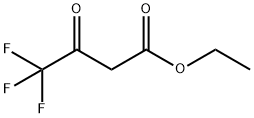
372-31-6
480 suppliers
$5.00/10g

31009-31-1
99 suppliers
$14.00/250mg
Yield:-
Reaction Conditions:
with hydrogenchloride;phosphoric acid in water;benzene
Steps:
275.1 Synthesis of 3-{(2S,4S)-4-[4-(8-fluoro-2-trifluoromethyl-4-quinolyl)-1-piperazinyl]-2-pyrrolidinylcarbonyl}-1,3-thiazolidine dihydrochloride
(1) 2-Fluoroaniline (10.0 g), ethyl trifluoroacetoacetate (16.6 g) and concentrated hydrochloric acid (0.1 mL) were dissolved in benzene (40 mL), and the mixture was refluxed in a reaction vessel equipped with Dean-Stark trap for 7 hr. The reaction mixture was concentrated under reduced pressure and 75% phosphoric acid (40 mL) was added thereto. The mixture was stirred at 110°C for 5 hr. The reaction mixture was added to water, neutralized with sodium hydrogencarbonate, and extracted with ethyl acetate. The extract was concentrated under reduced pressure to give 8-fluoro-4-hydroxy-2-trifluoromethylquinoline (1.77 g).
References:
WELFIDE CORPORATION EP1308439, 2003, A1
