8-CHLORO-1,2,3,4-TETRAHYDROCARBAZOLE synthesis
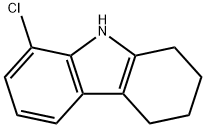
- Product Name:8-CHLORO-1,2,3,4-TETRAHYDROCARBAZOLE
- CAS Number:53475-34-6
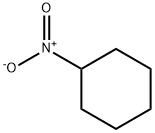
- Molecular formula:C12H12ClN
- Molecular Weight:205.68
Yield:53475-34-6 94%
Reaction Conditions:
with 1,3-bis(3-sulfopropyl)-1H-imidazol-3-ium trifluoromethanesulfonate in lithium hydroxide monohydrate at 100; for 0.333333 h;Microwave irradiation;Green chemistry;Fischer Indole Synthesis;
Steps:
3 4.3 Representative procedure for the one-pot Fischer indole synthesis
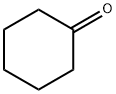
General procedure: Cyclohexanone (0.91 g, 10.0 mmol) was mixed with [(HSO3- p)2im][CF3SO3] (0.5 mmol) in water (15 mL), and phenylhydrazine hydrochloride (1.44 g, 10.0 mmol) was added. The mixture was then stirred at 100 8C for about 15 min under microwave irradiation. Reaction progress was monitored by GC-MS. After completion, the reaction mixture was cooled to room temperature, and 1,2,3,4-tetrahydrocarbazole was obtained by filtration. The remaining mixture of [(HSO3-p)2im][CF3SO3]/H2O was reused directly.
References:
Li, Bai Lin;Zhong, Ai Guo;Xu, Dan-Qian [Journal of Fluorine Chemistry,2012,vol. 144,p. 45 - 50,6]