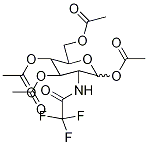
1,3,4,6-Tetra-O-acetyl-2-deoxy-2-trifluoracetamido-D-glucose synthesis
- Product Name:1,3,4,6-Tetra-O-acetyl-2-deoxy-2-trifluoracetamido-D-glucose
- CAS Number:7139-63-1
- Molecular formula:C16H20F3NO10
- Molecular Weight:443.3259
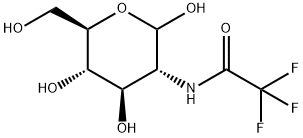
667462-08-0
1 suppliers
inquiry
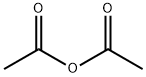
108-24-7
0 suppliers
$14.00/250ML

7139-63-1
19 suppliers
$125.00/250mg
![α-D-Glucopyranose, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 1,3,4,6-tetraacetate](/CAS/20210305/GIF/25258-08-6.gif)
25258-08-6
0 suppliers
inquiry
Yield:42.857 % de
Reaction Conditions:
with pyridine at 0 - 20; for 22 h;Inert atmosphere;Overall yield = 99 %; Overall yield = 10.9 g;
Steps:
2-Deoxy-1,3,4,6-tetra-O-acetyl-2-trifluoroacetamido-D-glucopyranose (2)1
Et3N (3.5 mL, 25.1 mmol) and ethyl trifluoroacetate (3.8 mL, 31.9 mmol) was addeddropwise to a solution of D-glucosamine hydrochloride (1) (5.38 g, 25.0 mmol) in MeOH(15 mL) at 0 °C, and then the mixture was stirred at room temperature for 15 h. Themixture was concentrated under reduced pressure, and the resulting residue was purifiedby column chromatography on silica gel (CHCl3/MeOH, 3:1~1:1) to give a 2:1.7 mixtureof 2-deoxy-2-trifluoroacetamido-d-glucopyranose (α/β = 1:1) and Et3N. Pyridine (50 mL)was added to the mixture and Ac2O (30.0 mL, 317 mmol) added dropwise at 0 C. Theresulting suspension was stirred at room temperature for 22 h. The mixture waspartitioned between EtOAc and H2O. The organic layer was washed with 1 M HCl, H2Oand brine, dried over Na2SO4, and concentrated under reduced pressure to give 2 (α/β =1:0.4) as a colorless solid (10.9 g, 99%);
References:
Kitamura, Yoshiaki;Moribe, Shuichi;Kitade, Yukio [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 19,p. 3174 - 3176] Location in patent:supporting information
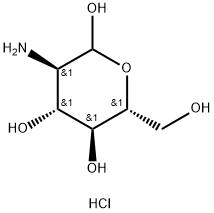
1078691-95-8
12 suppliers
inquiry

7139-63-1
19 suppliers
$125.00/250mg
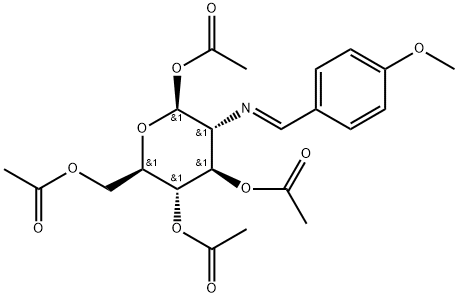
7597-81-1
37 suppliers
$14.00/250mg

7139-63-1
19 suppliers
$125.00/250mg
![6-(hydroxymethyl)-3-[(4-methoxyphenyl)methylideneamino]oxane-2,4,5-tri ol](/CAS/GIF/51471-40-0.gif)
51471-40-0
40 suppliers
$28.60/50MG

7139-63-1
19 suppliers
$125.00/250mg
![6-(hydroxymethyl)-3-[(4-methoxyphenyl)methylideneamino]oxane-2,4,5-tri ol](/CAS/GIF/51471-40-0.gif)
51471-40-0
40 suppliers
$28.60/50MG

7139-63-1
19 suppliers
$125.00/250mg