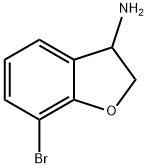
7-bromo-2,3-dihydro-1-benzofuran-3-amine synthesis
- Product Name:7-bromo-2,3-dihydro-1-benzofuran-3-amine
- CAS Number:1019631-11-8
- Molecular formula:C8H8BrNO
- Molecular Weight:214.06
1258400-12-2
20 suppliers
$186.00/100mg

1019631-11-8
9 suppliers
$688.00/1g
Yield:-
Reaction Conditions:
with ammonia in water;
Steps:
68
Reference Example 687-bromo-2, 3-dihydro-l-benzofuran-3-amine; [0397]A mixed solution of 7-bromo-l-benzofuran-3 (2H) -one (0.521 g, 2.45 mmol) , 0-methylhydroxyammonium chloride (0.306 g, 3.67 mmol) and sodium acetate (0.301 g, 3.67 mmol) in methanol (12 mL) was heated under reflux for 10 hr, and the mixture was stirred at room temperature for 16 hr. The reaction mixture was concentrated' under reduced pressure, the residue was poured into saturated aqueous sodium hydrogen carbonate, and the mixture was extracted with ethyl acetate. The extract was washed with saturated brine, dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane: ethyl acetate = 100:0 - 80:20) to give 7-bromo-l-benzofuran-3 (2H) -one O- methyloxime (0.468 g, yield 79%) as a yellow solid. To a solution of 7-bromo-l-benzofuran-3 (2H) -one O-methyloxime (0.468 g, 1.93 mmol) obtained above in tetrahydrofuran (9 mL) was slowly added dropwise borane-tetrahydrofuran solution (1 M, 5.80 mL, 5.80 mmol) at room temperature, and the mixture was heated under reflux under a nitrogen atmosphere for 3 hr. The reaction mixture was cooled, ice water was slowly added, 1 M hydrochloric acid was added, and the mixture was stirred at8O0C for 1.5 hr. The reaction mixture was allowed to cool, 28% aqueous ammonia solution was added to alkalify the solution, and the mixture was extracted with ethyl acetate. The extract was dried over sodium sulfate, and concentrated under reduced pressure. The residue (0.402 g) was dissolved in diethyl ether (4 mL) , and 4 M hydrochloric acid-ethyl acetate solution (0.480 mL) was slowly added. The precipitated solid was collected by filtration, and washed with diethyl ether to give 7-bromo-2, 3-dihydro-l-benzofuran-3-amine hydrochloride (0.434 g) as a beige solid. The solid obtained above was dissolved in28% aqueous ammonia solution, and the mixture was extracted with ethyl acetate. The extract was washed with saturated brine, dried over sodium sulfate, and concentrated under reduced pressure to give the title compound (0.366 g, yield 89%) as a yellow solid.1H NMR (300 MHz, DMSO-d6) δ 2.15 (2H, br s), 4.05-4.16 (IH, m) , 4.58-4.72 (2H, m) , 6.78-6.87 (IH, m) , 7.28-7.38 (2H, m) .
References:
WO2010/143733,2010,A1 Location in patent:Page/Page column 160-161