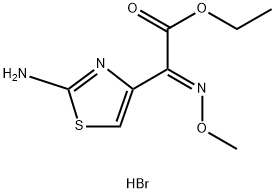
(E)-Ethyl 2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetate Hydrobromide synthesis
- Product Name:(E)-Ethyl 2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetate Hydrobromide
- CAS Number:69689-85-6
- Molecular formula:C8H12BrN3O3S
- Molecular Weight:310.17
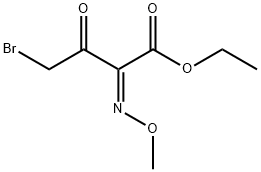
65872-39-1
3 suppliers
inquiry

69689-85-6
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with thiourea in ethanol;
Steps:
8.3 Preparation 8
(3) A solution of ethyl 2-methoxyimino-4-bromoacetoacetate (a mixture of syn and anti isomers) (17.4 g.) and thiourea (5.4 g.) in ethanol (100 ml.) was refluxed for 4 hours. The reaction mixture was allowed to stand and cooled in refrigerator to precipitate crystals. The crystals were collected by filtration, washed with ethanol and dried to give ethyl 2-methoxyimino-2-(2-amino-1,3-thiazol-4-yl)acetate hydrobromide (anti iosmer) (9.5 g.). The filtrate and the washings were put together and concentrated under reduced pressure. Water (100 ml.) was added to the residue and the mixture was washed with ether.
References:
US4279818,1981,A