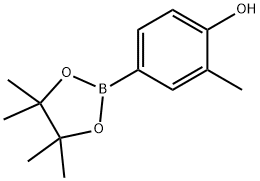
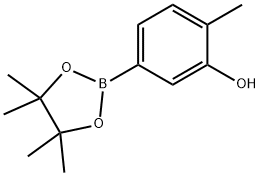
2-Methyl-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenol synthesis
- Product Name:2-Methyl-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenol
- CAS Number:627906-52-9
- Molecular formula:C13H19BO3
- Molecular Weight:234.1
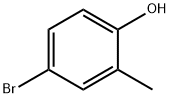
2362-12-1
195 suppliers
$5.00/1g
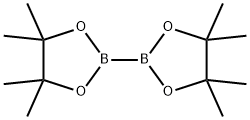
73183-34-3
591 suppliers
$6.00/5g

627906-52-9
44 suppliers
$132.00/100mg
Yield: 75%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 90;Inert atmosphere;
Steps:
2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenol (97)
General procedure A: Potassium acetate (2.94 g, 30.0 mmol), bis-(pinacolato)-diboron (3.05 g, 12.0 mmol) and bis(diphenylphosphine) ferrocene dichloropalladium (II) complex with (0355) dichloromethane (0.36 g, 0.5 mmol) was added to an anhydrous solution of 4-bromo-2-methylphenol (1.87 g, 10.0 mmol) in dioxane (180 mL) under anhydrous conditions in an atmosphere of nitrogen. The mixture was stirred at 90 °C overnight. The reaction was then quenched with water and extracted with ethyl acetate The combined organic layers were dried over magnesium sulfate, filtered and concentrated. The residue was purified by flash column chromatography on silica gel (eluent: dichloromethane/ethyl acetate, 10-100%) to give the title compound (12) as a white solid (1.75 g, 75%). ESI-MS m/z: 235.1508 [M+H]+; 1H NMR (400 MHz, CDC13) δ 7.60 (s, 1H), 7.55 (d, J= 7.9 Hz, 1H), 6.76 (d, J= 7.9 Hz, 1H), 4.97 (s, 1H), 2.25 (s, 3H), 1.33 (s, 12H); 13C NMR (101 MHz, CDC13) δ 156.77, 138.04, 134.46, 123.20, 114.56, 83.71, 24.99, 15.56.
References:
THE REGENTS OF THE UNIVERSITY OF COLORADO, A BODY CORPORATE;YIN, Hang Hubert;ZHANG, Shuting;HU, Zhenyi WO2019/89648, 2019, A1 Location in patent:Page/Page column 49-50
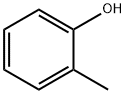
95-48-7
459 suppliers
$17.00/25g

627906-52-9
44 suppliers
$132.00/100mg

95-48-7
459 suppliers
$17.00/25g

627906-52-9
44 suppliers
$132.00/100mg
331273-58-6
30 suppliers
$67.00/500mg