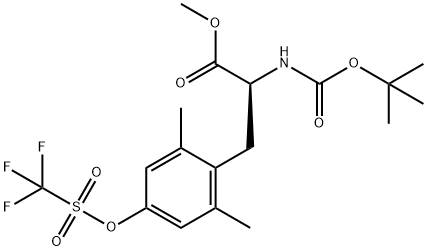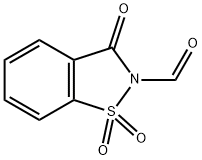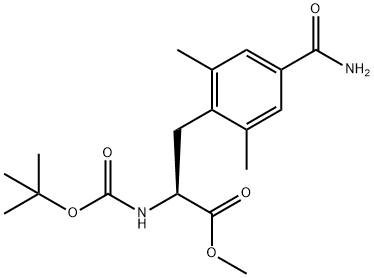
4'-carbamoyl N-Boc-2',6'-dimethyl-L-phenylalanine methyl ester synthesis
- Product Name:4'-carbamoyl N-Boc-2',6'-dimethyl-L-phenylalanine methyl ester
- CAS Number:623950-05-0
- Molecular formula:C18H26N2O5
- Molecular Weight:350.41
Yield:623950-05-0 100%
Reaction Conditions:
Stage #1: methyl (S)-2-((tert-butoxycarbonyl)amino)-3-(2,6-dimethyl-4-(((trifluoromethyl)sulfonyl)oxy)phenyl)propanoate;3-oxobenzo[d]isothiazole-2(3H)-carbaldehyde 1,1-dioxidewith potassium fluoride;palladium diacetate;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in N,N-dimethyl-formamide at 80 - 85; for 0.16 h;
Stage #2: with ammonia;triethylamine at 0 - 5;Reagent/catalyst;
Steps:
2 Preparation of methyl (S)-2-((tert-butoxycarbonyl)amino)-3-(4-carbamoyl-2,6- dimethylphenyl)propanoate, compound of formula (5) wherein n is 2, R1and R2are methyl, R5and R10are H, and Pg is tert- butyloxycarbonyl.
This example is representative of operations e.1 ) and e.2) of the process of the invention.The oily residue prepared as described in example 1 (6.0 mmol theoretical) was dissolved in /V,/V-dimethyl formamide (56 ml_). /V-formylsaccarin (1 .90 g, 9.0 mmol), Xantphos (0.16 g, 0.3 mmol), potassium fluoride (8.70 g, 15.0 mmol), and palladium (II) acetate (0.04 g, 0.2 mmol) were added. The resulting mixture was subjected to 3 cycles of vacuum/nitrogen, then maintained under stirring at 80-85 °C until complete conversion (about 16 hours). The reaction was cooled to 20-25 °C and subjected to 3 cycles of vacuum nitrogen (in order to remove the formed carbon monoxide) then it was further cooled to 0-5 °C and triethylamine (2.43 g, 24.0 mmol) and ammonia (0.15 g, 9.0 mmol) were added thereto. When the conversion was complete, the mixture was evaporated under reduced pressure in order to remove most of L/,/V-di methyl formamide, then water (42 ml.) and isopropyl acetate (8.5 ml.) were added. The mixture was heated to 45 °C, then filtered on a pad of celite and charcoal. The phases were separated, then a 5% (w/w) aqueous solution of sodium carbonate (4.80 g) was added to the organic phases. After having separated the resulting layers, the organic phase is concentrated under reduced pressure so as to obtain an oily residue (2.85 g, quantitative yield).
References:
WO2019/197274,2019,A1 Location in patent:Page/Page column 31-32; 36-37
![2-Propenoic acid, 3-[4-(aminocarbonyl)-2,6-dimethylphenyl]-2-[[(1,1-dimethylethoxy)carbonyl]amino]-, methyl ester, (2Z)-](/CAS/20180703/GIF/864825-84-3.gif)
864825-84-3
13 suppliers
inquiry

623950-05-0
18 suppliers
inquiry
1227311-10-5
12 suppliers
inquiry

623950-05-0
18 suppliers
inquiry
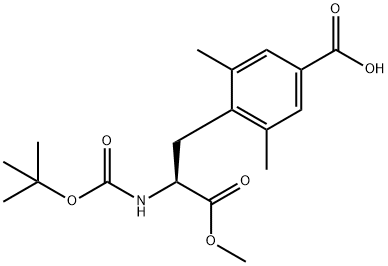
623950-04-9
2 suppliers
inquiry

623950-05-0
18 suppliers
inquiry
1206679-91-5
48 suppliers
inquiry
93267-04-0
249 suppliers
$5.00/250mg

623950-05-0
18 suppliers
inquiry