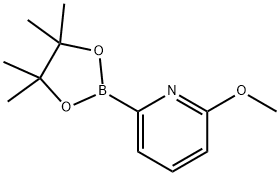
6-METHOXYPYRIDINE-2-BORONIC ACID PINACOL ESTER synthesis
- Product Name:6-METHOXYPYRIDINE-2-BORONIC ACID PINACOL ESTER
- CAS Number:1034297-69-2
- Molecular formula:C12H18BNO3
- Molecular Weight:235.09
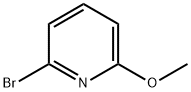
40473-07-2
279 suppliers
$7.00/1g
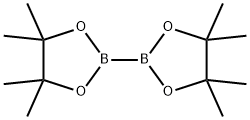
73183-34-3
581 suppliers
$6.00/5g

1034297-69-2
87 suppliers
$20.00/100mg
Yield:-
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in dimethyl sulfoxide at 80; for 2 h;
Steps:
A
To a mixture of 2-methoxy-6-bromopyridine (374 mg; 2.0 mmol) and bis(pinacolato) diboron (1.1 g; 4.0 mmol) in dimethylsulfoxide (degassed, 10 ml_) was added [1 ,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(ll) (82mg; 0.1 mmol) and potassium acetate (0.60 g; 6.0 mmol). The mixture was heated at 800C under a nitrogen atmosphere for 2 hours. The reaction mixture was poured into water (150 ml_) with stirring to give a dark precipitate, which was collected by filtration, washed with water, and dried under reduced pressure. The crude product was used without further purification. 1H (400MHz, CDCI3) 7.57 (1 H, dd), 7.44 (1 H, d), 6.78 (1 H, d), 4.02 (3H, s), 1.36 (12H, s).
References:
CANCER RESEARCH TECHNOLOGY LIMITED WO2008/74997, 2008, A1 Location in patent:Page/Page column 88