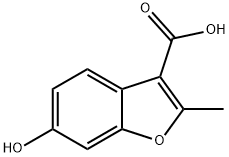
6-HYDROXY-2-METHYLBENZOFURAN-3-CARBOXYLIC ACID synthesis
- Product Name:6-HYDROXY-2-METHYLBENZOFURAN-3-CARBOXYLIC ACID
- CAS Number:854515-52-9
- Molecular formula:C10H8O4
- Molecular Weight:192.17
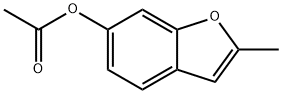
92810-82-7
2 suppliers
inquiry

854515-52-9
52 suppliers
$170.00/100mg
Yield: 93%
Reaction Conditions:
Stage #1:6-acetoxy-2-methylbenzofuran with aluminum (III) chloride;oxalyl dichloride in dichloromethane at 0 - 20; for 2.33333 h;
Stage #2: with methanol in dichloromethane at 0;
Stage #3: with potassium carbonate in methanol at 20; for 16 h;
Steps:
33.3
Step 3 6-hvdroxy-2-metfayl-benzofuran-3-carboxylic acidTo a slurry of aluminum trichloride (20.0 g, 150 mmol) in dichloromethane (200 mL) is added oxalyl chloride (13.0 mL, 150 mmol). The mixture is stirred at 0 0C for 30 minutes. A solution of acetic acid 2-methyl-benzofuran-6-yl ester (9.50 g; 49.9 mmol) in dichloromethane (50 mL) is added over 10 minutes. The ice-bath is removed and the reaction is stirred at room temperature for 2 hours. The reaction mixture is cooled to 0 0C and quenched with MeOH (50 mL). The mixture is concentrated to a residue under reduced pressure, dissolved in methanol (250 mL), and treated with potassium carbonate (8.28 g, 59.9 mmol). The mixture is stirred at room temperature for 16 hours, filtered through a pad of diatomaceous earth, and concentrated under reduced pressure. The residue is diluted with water (100 mL) and extracted with EtOAc (250 mLx2). The combined organic layers are dried over Na2SO^ filtered, and concentrated under reduced pressure. The crude product is purified by flash chromatography eluting with 25% EtOAc/Hexanes. The appropriate fractions are combined and concentrated under reduced pressure to afford the title compound (9.56 g, 93%). MS: 207.0 (M+l); 205.0 (M-I).
References:
ELI LILLY AND COMPANY WO2007/92751, 2007, A2 Location in patent:Page/Page column 27
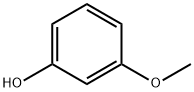
150-19-6
341 suppliers
$6.00/5g

854515-52-9
52 suppliers
$170.00/100mg
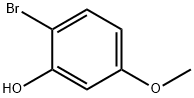
63604-94-4
140 suppliers
$7.00/250mg

854515-52-9
52 suppliers
$170.00/100mg
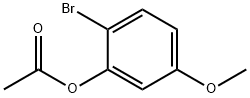
328945-89-7
1 suppliers
inquiry

854515-52-9
52 suppliers
$170.00/100mg