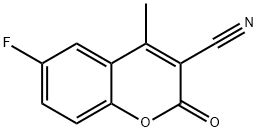
6-FLUORO-4-METHYLCOUMARIN-3-CARBONITRIL& synthesis
- Product Name:6-FLUORO-4-METHYLCOUMARIN-3-CARBONITRIL&
- CAS Number:288399-90-6
- Molecular formula:C11H6FNO2
- Molecular Weight:203.17
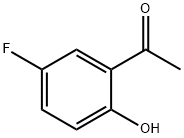
394-32-1
349 suppliers
$9.00/5g
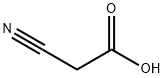
372-09-8
370 suppliers
$24.73/10gm:

288399-90-6
20 suppliers
$75.00/500mg
Yield:288399-90-6 92%
Reaction Conditions:
with 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide;triethylamine in acetic acid butyl ester;ethyl acetate at 120;Perkin condensation;
Steps:
General procedure: T3P mediated synthesis of coumarins: To a mixture of 2-hydroxyaryl aldehyde/ketone (1, 0.01 mol) and appropriate acetic acid (2, 0.01 mol) in n-BuOAc (10 mL) was added T3P (0.02 mol, 50% soln in EtOAc) followed by triethylamine (0.02 mol). The resulting reaction mixture was stirred at 120 °C for 6-10 h under conventional heating. When the reaction was completed (monitored by TLC), the mixture was cooled and washed with saturated NaHCO3 solution (1 × 10 mL), water and brine. The organic phase was dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the crude product was passed through a small plug of silica to afford the coumarins (3) in good purity and yield.
References:
Augustine, John Kallikat;Bombrun, Agnes;Ramappa, Balakrishna;Boodappa, Chandrakantha [Tetrahedron Letters,2012,vol. 53,# 33,p. 4422 - 4425] Location in patent:experimental part

394-32-1
349 suppliers
$9.00/5g
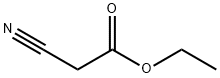
105-56-6
442 suppliers
$10.00/5ml

288399-90-6
20 suppliers
$75.00/500mg