
6-CHLORO-5-FLUOROBENZIMIDAZOLE-2-THIOL synthesis
- Product Name:6-CHLORO-5-FLUOROBENZIMIDAZOLE-2-THIOL
- CAS Number:101337-92-2
- Molecular formula:C7H4ClFN2S
- Molecular Weight:202.64
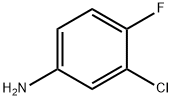
367-21-5
525 suppliers
$5.00/25g
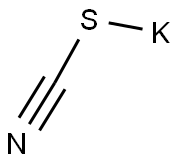
333-20-0
460 suppliers
$13.50/100G

101337-92-2
17 suppliers
inquiry
Yield:101337-92-2 89.31%
Reaction Conditions:
with bromine;acetic acid at 0 - 20; for 13.75 h;
Steps:
Synthesis of 5-Chloro-6-fluoro-benzothiazol-2-amine (5)
To a pre-cooled glacial acetic acid (40ml), 0.416 mole of potassium thiocyanate and 0.05 mole of 3-chloro-4-fluoro aniline was added. The mixture was placed in freezing mixture of ice and salt and mechanically stirred while 6 ml of bromine in 24 ml of glacial acid was added from a dropping funnel maintaining the temperature0oC. After all the bromine has been added(105 min.), the solution was stirred for an additional 2 hours at 0oC and at room temperature for 10 hours. It was allowed to stand overnight during which an orange precipitate settled at the bottom, water(30 ml) was added quickly and slurry was heated at 85oC on a steam bath and filtered hot. The orange residue was placed in a reaction flask and treated with 10 ml of glacial acetic acid, heated again to85oC and filtered hot. The combined filtrate was cooled and neutralized with concentrated ammonia solution to pH 6 when a dark yellow precipitate was collected. Recrystallisation from ethanol and water mixture. The product was obtained as pale-yellow crystals. Yield: 89.31%; m.p: 168 °C; I.R. (KBr, cm-1) 691, 791, 1385, 1638, 2920, 3058, 3249, 3433; 1H-NMR (300 MHz, DMSO-d6): δ (in ppm)= 2.51 (DMSO), 4.82 (s, -NH2), 7.88 (d, Ar-H), 8.20 (d, Ar-H). Anal. Calc. for C7H4N2ClFS: C 41.49, H 1.99, N 13.82; Found: C 41.48, H 1.93, N 13.87.
References:
Naqvi, Arshi [Oriental Journal of Chemistry,2018,vol. 34,# 6,p. 3134 - 3139]

367-21-5
525 suppliers
$5.00/25g

333-20-0
460 suppliers
$13.50/100G

101337-92-2
17 suppliers
inquiry

101337-93-3
5 suppliers
inquiry
154371-25-2
52 suppliers
$39.00/1mg

101337-92-2
17 suppliers
inquiry