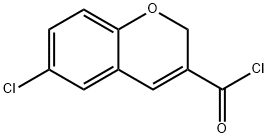
6-CHLORO-2H-1-BENZOPYRAN-3-CARBONYL CHLORIDE synthesis
- Product Name:6-CHLORO-2H-1-BENZOPYRAN-3-CARBONYL CHLORIDE
- CAS Number:306935-54-6
- Molecular formula:C10H6Cl2O2
- Molecular Weight:229.06
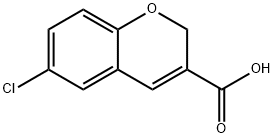
83823-06-7
97 suppliers
$34.00/500mg

306935-54-6
39 suppliers
$60.09/1gm:
Yield:306935-54-6 100%
Reaction Conditions:
with thionyl chloride;N,N-dimethyl-formamide in toluene at 60 - 70; for 3 h;Product distribution / selectivity;Heating / reflux;
Steps:
1
6-Chloro-2H-chromene-3-carboxylic acid [5-chloro-2-(1H-tetrazol-5-yl)-phenyl]-amide (22)To a solution of commercial 5-chloro(2H)-1 -benzopyran-3-carboxylic acid (0.40 g, 1 eq) in TOL dry (20 ml), thionyl chloride (2.77 ml, 20 eq) is added, followed by 1 -2 drops of dry DMF. The resulting yellow solution is refluxed for 3 hours and evaporated to dryness, to get the correspondent 6-chloro-2H-chromene-3-carbonyl chloride (yield -100%). To a solution of 5-chloro-2-(1 H-tetrazol-5-yl)-phenylamine prepared as described by Valgeirsson et al. in Journal of Medicinal Chemistry 2004 47 (27) 6948-6957 in dry TOL (15 ml) and Py (1.5 ml, -10 eq), a solution of the above acid chloride derivative (1 eq) in dry TOL is added drop wise, and the resulting mixture is stirred at room temperature overnight. The day after the suspension is evaporated to dryness (-0.73 g, -100% yield). The crude compound is easily purified by crystallization from EtOH. LC-ESI-HRMS of [M-H]- shows 386.0197 Da. CaIc. 386.021156 Da, dev. -3.8 ppm. 6-Chloro-2H-chromene-3-carboxylic acid [4-bromo-2-(1H-tetrazol-5-yl)-phenyl]-amide (24)A mixture of commercial 5-chloro(2H)-1 -benzopyran-3-carboxylic acid (0.5 g, 1 eq) and an excess of thionyl chloride (5.6 ml) in dry TOL (20 ml) is heated (60- 70°C) until the starting material disappears completely. The mixture is then evaporated to dryness and the residue is taken up in DCM and washed with aqueous NaHCO3. To the residue of the organic phase, dried over magnesium sulphate and evaporated to dryness, TOL and an equimolar amount of 4-bromo-2-(1 H-tetrazol-5-yl)- phenylamine prepared as described by in US 2002037905 (0.95 g) in a mixture of anhydrous TOL (20 ml) and Py (2 ml) is added and stirring is continued overnight. The solvent is evaporated in vacuo and the residue is suspended in diluted HCI. The residue is collected with by filtration, washed with water, dried (0.80 g, -80% yield) and purified by crystallisation from EtOH. LC-ESI-HRMS of [M+H]+ shows 431 .9869 Da. CaIc. 431 .986291 Da, dev. 1 .4 ppm.
References:
WO2008/74755,2008,A2 Location in patent:Page/Page column 31; 32
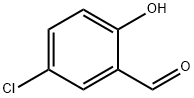
635-93-8
359 suppliers
$6.00/5g

306935-54-6
39 suppliers
$60.09/1gm:
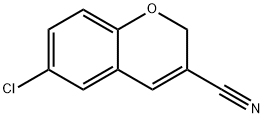
57543-67-6
55 suppliers
inquiry

306935-54-6
39 suppliers
$60.09/1gm:

83823-19-2
0 suppliers
inquiry

306935-54-6
39 suppliers
$60.09/1gm: