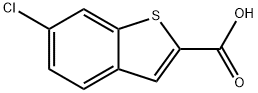
6-CHLORO-1-BENZOTHIOPHENE-2-CARBOXYLIC ACID synthesis
- Product Name:6-CHLORO-1-BENZOTHIOPHENE-2-CARBOXYLIC ACID
- CAS Number:26018-73-5
- Molecular formula:C9H5ClO2S
- Molecular Weight:212.65
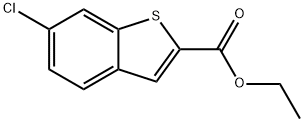
![Methyl 6-chlorobenzo[b]thiophene-2-carboxylate](/StructureFile/ChemBookStructure22/GIF/CB9956809.gif)
104795-85-9
51 suppliers
$77.00/250mg

26018-73-5
76 suppliers
$19.00/100mg
Yield:26018-73-5 98%
Reaction Conditions:
Stage #1: methyl 6-chlorobenzo[b]thiophene-2-carboxylatewith lithium hydroxide;lithium hydroxide monohydrate in tetrahydrofuran at 20; for 4 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;lithium hydroxide monohydrate; pH=2 - 3;
Steps:
S13 Preparation of (6-chloro-benzo[b]thiophen-2-ylmethyl)-[2-(3,4-dichloro-phenyl)-ethyl]-amine
To a solution of 600 mg (3.23 mmol) of 4-chloro-2-nitro-benzaldehyde in DMF (7 ml) were added 559 mg (4.04 mmol) of potassium carbonate and 294 μl (3.23 mmol) of methyl thioglycolate at 0° C. The reaction mixture was stirred for 30 min at 0° C. and then for 24 h at RT. Then the mixture was poured into ice-water and the precipitate was collected by filtration and dissolved in ethyl acetate. The solution was dried (MgSO4) and concentrated to yield 630 mg (86%) of 6-chloro-benzo[b]thiophene-2-carboxylic acid methyl ester as a white solid. 1HNMR (CDCl3, 300 MHz): δ 3.95 (s, 3H), 7.88 (dd, J=8.6 and 1.9 Hz, 1H), 7.79 (d, J=8.6 Hz, 1H), 7.85 (d, J=1.9 Hz, 1H), 8.02 (s, 1H).To a solution of 630 mg (2.78 mmol) of 6-chloro-benzo[b]thiophene-2-carboxylic acid methyl ester in THF (5 ml) were added 6.95 ml of 1N LiOH-solution and the reaction mixture was stirred at RT for 4 h. The pH was then adjusted to 2-3 by addition of 1N HCl and the mixture was extracted with ethyl acetate. The combined organic extracts were washed with brine, dried (MgSO4) and concentrated to yield 579 mg (98%) of 6-chloro-benzo[b]thiophene-2-carboxylic acid as white solid. MS (ISP) 211.0 (M-H)-.In analogy to example S3 (steps a to b) 139 mg (0.65 mmol) of 6-chloro-benzo[b]thiophen-2-carboxylic acid were converted into 52 mg (0.14 mmol) of (6-chloro-benzo[b]thiophen-2-ylmethyl)-[2-(3,4-dichloro-phenyl)-ethyl]-amine. The product was obtained as colorless liquid. MS (ISP) 370.0 (M+H)+.
References:
US2007/185113,2007,A1 Location in patent:Page/Page column 13
105191-53-5
24 suppliers
$489.00/5g

26018-73-5
76 suppliers
$19.00/100mg
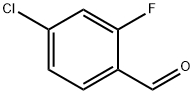
61072-56-8
288 suppliers
$14.00/5g

26018-73-5
76 suppliers
$19.00/100mg