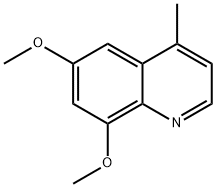
6,8-DIMETHOXY-4-METHYLQUINOLINE synthesis
- Product Name:6,8-DIMETHOXY-4-METHYLQUINOLINE
- CAS Number:51049-14-0
- Molecular formula:C12H13NO2
- Molecular Weight:203.24

38771-21-0
139 suppliers
$22.00/1g
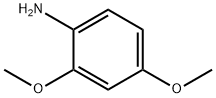
2735-04-8
157 suppliers
$25.00/25g

51049-14-0
52 suppliers
$90.00/100mg
Yield:51049-14-0 20%
Reaction Conditions:
with sodium tetrachloroaurate(III) dihyrate in ethanol at 70; for 24 h;
Steps:
General procedure for the alkylation/gold-catalyzed annulation reactions of anilines with propargylic bromide derivatives: to a solution of aniline 1 (2.0 mmol) in absolute ethanol (2 mL) were added propargylic bromide 2 (0.66 mmol) and NaAuCl4·2H2O (0.05 mmol). The resulting mixture was heated at 70 °C for 24 h. The reaction was monitored by TLC. After cooling, the solvent was concentrated under reduced pressure. The residue was dissolved in ethyl acetate and extracted three times with a saturated solution of NaHCO3. The combined aqueous extracts were extracted three times with ethyl acetate. The combined organic extracts were dried (Na2SO4) and evaporated under reduced pressure. The residue was purified by flash chromatography (silica gel, n-hexanes-ethyl acetate mixtures) to give quinoline 4.
References:
Alfonsi, Maria;Arcadi, Antonio;Chiarini, Marco;Marinelli, Fabio [Tetrahedron Letters,2011,vol. 52,# 40,p. 5145 - 5148] Location in patent:experimental part