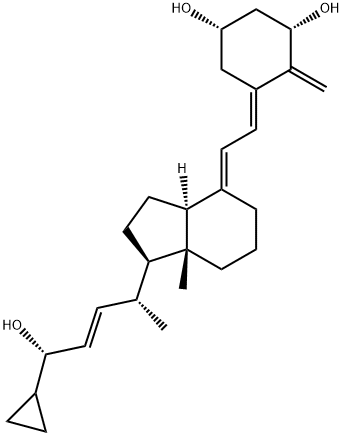
(5E)-Calcipotriene synthesis
- Product Name:(5E)-Calcipotriene
- CAS Number:113082-99-8
- Molecular formula:C27H40O3
- Molecular Weight:412.6
Yield:-
Reaction Conditions:
with tetrabutyl ammonium fluoride in tetrahydrofuranProduct distribution / selectivity;Heating / reflux;
Steps:
10.1.1; 10.1.2
EXAMPLE 10; Preparation of Compound 1(a, b); (10-1-1) Preparation of Compound 1(a)(1α,3β,5Z,7E,22E,24S) and 1(a),(1β,3β,5Z,7E,22E,24S); Compound 9(a) (200 g, 0.38 mol) (24S,Z=t-BuMe2SiO) is tetra-n-butylammonium fluoride (0.36 kg, 1.14 mol ) are dissolved in THF (2.7 L). Then the mixed solution is heated to reflux. After the reaction is complete, the reaction solution is concentrated. The residue after concentration is added with ethyl acetate and water for extraction. The organic layer is washed, concentrated to give a crude product, which is purified through a chromatographic column to give compound 11(a) [1(α,β),3β,5E,7E,22E,24S] (130 g) is obtained. The compound 11(a) [1(α,β),3β,5E,7E,22E,24S](130 g) is added to a mixture of ethyl acetate (130 mL) and water (520 mL). The mixture is stirred for 1 hour at room temperature. Then the mixture is filtered and a solid product 11(a) [1(α,β),3β,5E,7E,22E,24S] (80 g) is obtained. The filtrate is separated and the organic layer is separated and concentrated to obtain a residue (50 g). The residue, and phenyl boronic acid (10 g, 82 mmol) are dissolved in dichloromethane (2 L). The reaction is processed for 3.5 hours. After the reaction is complete, the reacted solution is concentrated and purified through a chromatographic column to give compound 11(a) [1α,3β,5E,7E,22E,24S] (15 g) and cyclic-1,3-boronate ester of 11(a) [1β,3β,5E,7E,22E,24S] (15 g). The compound 11(a) [1α,3β,5E,7E,22E,24S] (61 g), and 9-acetylanthracene (6 g) are dissolved in acetone (10 L). The acetone solution is photolysis at a temperature less than 10° C. in an atmosphere of argon. After the reaction is complete, the reaction mixture is concentrated and purified through a chromatographic column to give a crude product (compound 1(a) [1α,3β,5E,7E,22E,24S] (60.1 g). Compound 1(a) [1α,3β,5E,7E,22E,24S]: λmax=266 nm. m/z: 412. 1H NMR (200 MHz, CDCl3) δ 0.54 (s, 3 H), 1.02 (d, 3H, J=8.25 Hz), 3.39-3.43(m, 1H), 4.20(br, 1H), 4.41(br, 1 H), 4.97(s, 1 H), 5.29(s, 1 H), 5.43-5.46 (m, 2 H),5.99 (d, 1H, J=14.0 Hz ),6.35 (d, 1 H, J=14.05 Hz). The cyclic-1,3-boronate ester of 11(a) [1β,3β,5E,7E,22E,24S] (10 g) produced is dissolved in ethyl acetate. Then hydrogen peroxide (10 ml) is added to the ethyl acetate solution to react for 1 hour. After the reaction is completed, the reaction mixture is concentrated and purified through a chromatographic column to give compound 11(a) [1β,3β,5E,7E,22E,24S] (5 g). The compound 11(a) [1β,3β,5E,7E,22E,24S] (5 g, 12.12 mmol) produced, and 9-acetylanthracene (0.5 g, 2.27 mmol) are dissolved in acetone in an atmosphere of argon. The acetone solution is photolysis at a temperature less than 10° C. After the reaction is completed, the reacted solution is concentrated and purified through a chromatographic column to give compound 1(a) [1β,3β,5E,7E,22E,24S]. Compound 1(a) [1β,3β,5E,7E,22E,24S]: λmax=266 nm. m/z: 412. 1H NMR (200 MHz, CDCl3) δ 0.54 (s, 3 H ), 1.0 (d, 3H, J=6.62 Hz), 3.36-3.43 (m, 1H), 4.07(m, 1H), 4.32 (br, 1H), 4.97(s,1H), 5.25(s, 1 H), 5.42-5.45 (m, 2 H), 6.02 (d, 1 H, J=11.24 Hz), 6.41(d, 1 H, J =11.24 Hz).; (10-1-2) Preparation of Compound 1(a)(1α,3β,5Z,7E,22E,24S); Compound 9(a) (287 g, 0.54 mol) (24S,Z=t-BuMe2SiO), tetra-n-butylammonium fluoride (344 g, 1.09 mol) are dissolved in THF (2.8 L). Then ,the mixture is heated to reflux. After the reaction is complete,1 the reaction mixture is concentrated. The residue after concentrated is added with ethyl acetate and water for extraction. The organic layer is washed, concentrated to give a crude product, which is purified through a chromatographic column to give compound 11(a) [1(α,β)3β,5E,7E,22E,24S] (139 g). The compound 11(a) [1(α,β)3β,5E,7E,22E,24S] (139 g, 0.34 mol), and 9-acetylanthracene (13.9 g, 63.10 mmol) are dissolved in acetone (20 L) in an atmosphere of argon. The mixture is photolysis at a temperature less than 10° C. After the reaction is complete, the reaction mixture is concentrated and purified through a chromatographic column to give compound 1(a) [1(α,β)3β,5E,7E,22E,24S] (60.1 g). The compound 1(a) [1(α,β)3β,5E,7E,22E,24S] and phenyl boronic acid (16 g, 0.13 mmol) are dissolved in acetone (5 L). The reaction is processed for 3 hours. After the reaction is complete, the reaction mixture is concentrated and purified through a chromatographic column to give compound 1(a) [1α,3β,5E,7E,22E,24S] (126 g) and cyclic-1,3-boronate ester of 1(a) [1β,3β,5E,7E,22E,24S].
References:
Formosa Laboratories, Inc. US2007/88007, 2007, A1 Location in patent:Page/Page column 10-11
![1,3-Cyclohexanediol, 5-[(2E)-2-[(1R,3aS,7aR)-1-[(1R,2E,4S)-4-cyclopropyl-4-hydroxy-1-methyl-2-buten-1-yl]octahydro-7a-methyl-4H-inden-4-ylidene]ethylidene]-4-methylene-, (1R,5E)-](/CAS/20210111/GIF/934338-38-2.gif)
934338-38-2
0 suppliers
inquiry
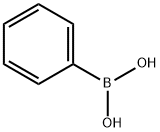
98-80-6
740 suppliers
$5.00/5g

113082-99-8
85 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

![Cyclohexanol, 3-[(2E)-2-[(1R,3aS,4E,7aR)-1-[(1R,2E,4S)-4-cyclopropyl-4-hydroxy-1-methyl-2-buten-1-yl]octahydro-7a-methyl-4H-inden-4-ylidene]ethylidene]-5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methylene-, (3E,5R)-](/CAS/20210111/GIF/934338-30-4.gif)