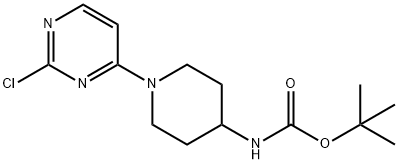
[1-(2-CHLORO-PYRIMIDIN-4-YL)-PIPERIDIN-4-YL]-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:[1-(2-CHLORO-PYRIMIDIN-4-YL)-PIPERIDIN-4-YL]-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:596817-49-1
- Molecular formula:C14H21ClN4O2
- Molecular Weight:312.8

3934-20-1
740 suppliers
$9.00/1g
73874-95-0
456 suppliers
$10.00/250mg
![[1-(2-CHLORO-PYRIMIDIN-4-YL)-PIPERIDIN-4-YL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/596817-49-1.gif)
596817-49-1
29 suppliers
$125.00/250mg
Yield:596817-49-1 73%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 16 h;
Steps:
437
A solution of 2,4-dichloropyrimidine (2.0 g, 13.4 mmol) and te/t-butyl piperidin-4 ylcarbamate (2,7 g, 13.9 mmol) in THF (50 mL) was charged with triethyl amine (2.8 mL, 20.1 mmol) at 0 °C. The reaction mixture was stirred at ambient temperature for 16 h. The reaction mixture was concentrated, and the residue was taken up in ethyl acetate; the organic layer was washed with NH4CI solution followed by brine; dried over sodium sulphate, filtered and concentrated. The crude obtained was purified by combi-flash companion (silica gel, CH3OH/CH2CI2) to provide feri-butyl (l-(2-chloropyrimidin-4- yl)piperidin-4-yl)carbamate 417k (2.4 g, 73%). NMR (400 MHz, CDC13): 0 8,01 (d, ./ 6.0 Hz, 1H), 6.41 (d, ./ 6.0 Hz, 1H), 4.63 (d, J= 6.4 Hz, 1H), 4 ,29 (br s, 2H), 3.74 (br s, 1H), 3 ,06 (t J = 12.0 Hz, 2H), 2,05 (br 2H), 1.51 (s, 9H), 1.40-1.33 (m, 2H); ESH-APCl MS m/z 313 [M + l l | .
References:
WO2017/58503,2017,A1 Location in patent:Page/Page column 419; 427; 428