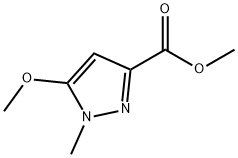
methyl 5-methoxy-1-methyl-1H-pyrazole-3-carboxylate synthesis
- Product Name:methyl 5-methoxy-1-methyl-1H-pyrazole-3-carboxylate
- CAS Number:58364-91-3
- Molecular formula:C7H10N2O3
- Molecular Weight:170.17
51985-95-6
115 suppliers
$16.00/100mg

74-88-4
349 suppliers
$15.00/10g

58364-91-3
19 suppliers
$9.00/100mg
Yield:58364-91-3 68.8%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 0 - 20;Inert atmosphere;
Steps:
134.134A
Example 134; (S, E)-Methyl 4-(6-(l-(3-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)acrylamido)-2-(5- methoxy-1-methyl-1H-pyrazol-3-yl)ethyl)-3-oxo-2>3-dihydropyridazin-4- yl)phenylcarbamate; 134 A. methyl 5 -methoxy-1 -methyl- 1H-pyrazole-3-carboxylate: To a cooled solution (0 °C) of methyl 5-hydroxy-1-methyl-1H-pyrazole-3-carboxylate (1.60 g, 10.25 mmol) and K2CO3 (2.124 g, 15.37 mmol) in DMF (10 mL) was added dropwise MeI (0.703 mL, 11.27 mmol). The reaction mixture was stirred under argon at rt for 5 days. The reaction mixture was diluted with EtOAc, washed with H2O (1 x 15 mL), saturated NaHCO3 (1 x 15 mL) and brine (1 x 15 mL). The organic phase was dried over MgSO^ filtered an Purification by normal phase
References:
WO2009/114677,2009,A1 Location in patent:Page/Page column 209-210