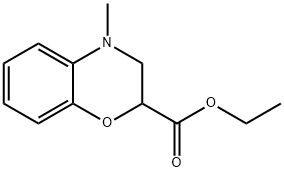
ETHYL 4-METHYL-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZINE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 4-METHYL-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZINE-2-CARBOXYLATE
- CAS Number:54442-28-3
- Molecular formula:C12H15NO3
- Molecular Weight:221.25
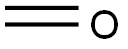
50-00-0
866 suppliers
$10.00/25g
![3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/22244-22-0.gif)
22244-22-0
51 suppliers
$45.00/100mg
![ETHYL 4-METHYL-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZINE-2-CARBOXYLATE](/CAS/GIF/54442-28-3.gif)
54442-28-3
16 suppliers
$244.00/500mg
Yield:54442-28-3 71%
Reaction Conditions:
Stage #1: formalin;2(R,S)-ethoxycarbonyl-3,4-dihydro-2H-1,4-benzoxazinewith glacial acetic acid in lithium hydroxide monohydrate at 20; for 0.166667 h;
Stage #2: with sodium cyanotrihydridoborate in lithium hydroxide monohydrate at 0 - 20; for 0.75 h;
Steps:
1.C Step C: ethyl 4-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazine-2-carboxylate
To a solution of the product of Step B (26 g, 125.46 mmol) in HOAc (100 mL) was added 60HCHO aqueous solution (25.10 g, 501.84 mmol) . The reaction mixture was stirred at rt for 10 min. Then the mixture was cooled to 0 , Sodium cyanoborohydride (15.77 g, 250.92 mmol) was added several portions for 15 min at 0 . The final solution was stirred at rt for 30 min. The resulting mixture was poured into ice water (100 mL) , neutralized by 5N NaOH aqueous solution till pH7, then extracted with EA (200 mL x 3) . The combined organic layer was washed by brine, dried over sodium sulfate anhydrous then concentrated and purified by silica gel column chromatography eluting with PE: EA5: 1to get the desired product (19.7 g, 71) as yellow oil.[0285]MS: M/e 222 (M+1)+
References:
WO2016/8411,2016,A1 Location in patent:Paragraph 0111

74-88-4
349 suppliers
$15.00/10g
![3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/22244-22-0.gif)
22244-22-0
51 suppliers
$45.00/100mg
![ETHYL 4-METHYL-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZINE-2-CARBOXYLATE](/CAS/GIF/54442-28-3.gif)
54442-28-3
16 suppliers
$244.00/500mg
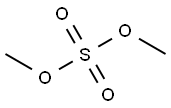
77-78-1
305 suppliers
$22.00/25g
![3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/22244-22-0.gif)
22244-22-0
51 suppliers
$45.00/100mg
![ETHYL 4-METHYL-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZINE-2-CARBOXYLATE](/CAS/GIF/54442-28-3.gif)
54442-28-3
16 suppliers
$244.00/500mg
![3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/22244-22-0.gif)
22244-22-0
51 suppliers
$45.00/100mg
![ETHYL 4-METHYL-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZINE-2-CARBOXYLATE](/CAS/GIF/54442-28-3.gif)
54442-28-3
16 suppliers
$244.00/500mg
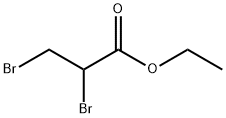
3674-13-3
230 suppliers
$17.00/25g
![ETHYL 4-METHYL-3,4-DIHYDRO-2H-BENZO[B][1,4]OXAZINE-2-CARBOXYLATE](/CAS/GIF/54442-28-3.gif)
54442-28-3
16 suppliers
$244.00/500mg