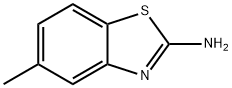
5-Methyl-2-aminobenzothiazole synthesis
- Product Name:5-Methyl-2-aminobenzothiazole
- CAS Number:14779-17-0
- Molecular formula:C8H8N2S
- Molecular Weight:164.23
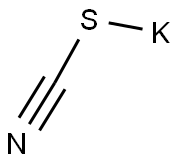
333-20-0
444 suppliers
$13.50/100G
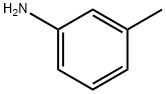
108-44-1
374 suppliers
$9.00/1g

14779-17-0
110 suppliers
$62.00/100mg
Yield: 92%
Reaction Conditions:
Stage #1:potassium thioacyanate;1-amino-3-methylbenzene with nano-BF3/SiO2 in acetonitrile for 0.5 h;Cooling with ice;
Stage #2: with bromine in acetonitrile at 0 - 20; for 5 h;
Steps:
General procedure for the synthesis of 2-aminobenzothiazoles catalyzedby nano-BF3/SiO2
General procedure: A solution of substituted aniline (2 mmol) in acetonitrile (15 ml) was added to a solution of KSCN (8 mmol) in acetonitrile (15 ml). Then, 0.06 g (30 mol % BF3) of nano-BF3/SiO2 was added to the mixture, then was placed in a freezing mixture of ice and salt and mechanically stirred for 30 min. Then, bromine (4 mmol, 0.2 ml) in acetonitrile (3 ml) as solvent was added from a dropping funnel at such a rate that the temperature never rose beyond 0°C. After all the bromine was added at 60 min, the solution was stirred for 4 h at room temperature. The progress of the reaction was monitored by TLC. Then, the mixture was poured into water with stirring and the mixture was heated to 70°C on a steam bath and filtered hot to remove the catalyst and the recovered catalyst was washed with acetone and reused in the reaction. The filtrate was neutralized with 10 % NaOH solution and the precipitate was collected on a filter, dried and recrystallized from ethanol (10 ml) to afford the corresponding products. All of the 2-aminobenzothiazole products were identified by physical and spectroscopic data as reported below, compared and contrasted with authentic samples. Spectral data for selected products 6-Bromo-1,3-benzothiazol-2-amine (2e) Yellow solid; Yield = 93 %; m.p. =202-204°C; (m.p. = 203°C), FT-IR (KBr)/t(cm-1): 3315, 3012, 2835,1580, 1476, 1261, 920, 742, 512. 1H NMR (400 MHz, CDCl3)/d ppm: 5.44 (s, 2H, NH2) 7.4-7.5 (d, 2H, Ar-H), 7.71 (s, 1H, Ar-H); 13C NMR/(100 MHz, DMSO-d6)/d ppm: 119, 120.9, 125.15, 126.07, 133.1, 152.15, 167.75.
References:
Naeimi, Hossein;Heidarnezhad, Arash [Research on Chemical Intermediates,2016,vol. 42,# 12,p. 7855 - 7868]
621-40-9
68 suppliers
$8.00/1g

14779-17-0
110 suppliers
$62.00/100mg
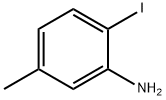
13194-69-9
112 suppliers
$5.00/250mg
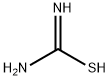
17356-08-0
3 suppliers
inquiry

14779-17-0
110 suppliers
$62.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

108-44-1
374 suppliers
$9.00/1g

14779-17-0
110 suppliers
$62.00/100mg

621-40-9
68 suppliers
$8.00/1g

14779-17-0
110 suppliers
$62.00/100mg
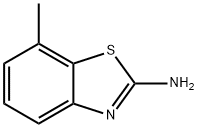
14779-18-1
33 suppliers
$232.00/250mg