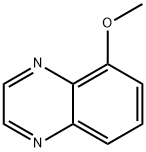
5-Methoxyquinoxaline synthesis
- Product Name:5-Methoxyquinoxaline
- CAS Number:19506-17-3
- Molecular formula:C9H8N2O
- Molecular Weight:160.17

131543-46-9
0 suppliers
inquiry
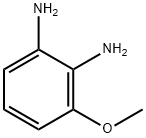
37466-89-0
96 suppliers
$30.00/100mg

19506-17-3
11 suppliers
inquiry
Yield:19506-17-3 71%
Reaction Conditions:
with sodium hydrogensulfite in water at 20; for 0.25 h;
Steps:
27 5-Methoxyquinoxaline:
At room temperature, a solution of oxaldehyde in H20 (40%, 4 mL) was added to a solution of 3-methoxybenzene-1,2-diamine (3.66 g, 26.53 minol) in water (100.00 mL). Then NaHSO3 (7.59 g, 72.94 minol) was added slowly. The resulting solution was stirred for 15 min at room temperature. When the reaction was done, the insoluable solids in the reactionminxture were filtered out. The filtrate was extracted with DCM (300 mL x 3), and the organic phases were combined, washed with brine and dried over Na2SO4. The solvent was removed under reduced pressure and the residue was purified by flash chromatography eluting with EtOAc in hexane (0% to 100% gradient) to yield 5-methoxyquinoxaline as dark red oil (3.02 g, 71%). MS: m/z = 161.0 [M+Hj.
References:
WO2017/106607,2017,A1 Location in patent:Paragraph 00460

37466-89-0
96 suppliers
$30.00/100mg
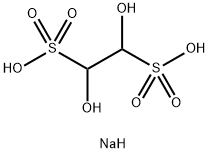
517-21-5
95 suppliers
$17.00/25g

19506-17-3
11 suppliers
inquiry
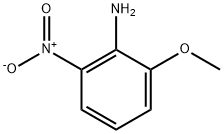
16554-45-3
140 suppliers
$16.00/1g

19506-17-3
11 suppliers
inquiry