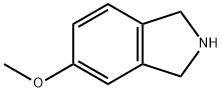
5-METHOXY-2,3-DIHYDRO-1H-ISOINDOLE synthesis
- Product Name:5-METHOXY-2,3-DIHYDRO-1H-ISOINDOLE
- CAS Number:127168-88-1
- Molecular formula:C9H11NO
- Molecular Weight:149.19
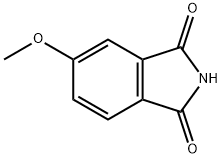
50727-04-3
55 suppliers
$23.00/100mg

127168-88-1
109 suppliers
$70.00/100mg
Yield: 47%
Reaction Conditions:
Stage #1:4-methoxyphthalimide with borane-THF in tetrahydrofuran at 0; for 16 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water for 3 h;Heating / reflux;
Steps:
C.C2
A stirred solution of 4-methoxyphthalimide (21.3 g, 0.12 mol) in anhydrous tetrahydrofuran (425 ml) at 00C was treated dropwise with a solution of borane in tetrahydrofuran (1M1 340 ml, 0.34 mol) and the resulting mixture was stirred and held at reflux for 16 hours. The mixture was cooled to 00C, methanol (150 ml) was added dropwise followed by 5M hydrochloric acid (150 ml) and the mixture was stirred and held at reflux for 3 hours. Upon cooling to room temperature the organic solvent was removed in vacuo, the mixture was diluted with water (750 ml) and was extracted with dichloromethane (3 x 750 ml). The aqueous layer was basified to pH 12 or above by the addition of 5M sodium hydroxide, extracted with dichloromethane (3 x 750 ml) and the combined extracts were evaporated to dryness in vacuo to afford 5-methoxyisoindoline (8.34 g, 47%) as a brown oil. 1H NMR (DMSOd6) 7.13 (1 H1 d), 6.84 (1H, d), 6.74 (1H1 dd), 4.05 (2H, s), 4.01 (2H, s), 3.73 (3H, s). MS: [M+H]+ 150.
References:
ASTEX THERAPEUTICS LIMITED WO2008/44029, 2008, A1 Location in patent:Page/Page column 204
127168-89-2
14 suppliers
$304.00/250mg

127168-88-1
109 suppliers
$70.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

127168-88-1
109 suppliers
$70.00/100mg
4685-47-6
105 suppliers
$10.00/5g

127168-88-1
109 suppliers
$70.00/100mg